Journal of
eISSN: 2373-6453


Liver acinar zonation allows for diverse functions to be performed on demand by specialized hepatocytes. Therefore, understanding how hepatitis C virus (HCV) infection may modulate the acinar zone phenotypes should provide insights into the pathophysiology of HCV infection. We assessed the effects HCV infection in the highly-differentiated primary human hepatocyte culture that we have developed for the efficient replication of intact HCV virions.
We found that HCV genotype 1 infection stimulated the zone-3 phenotype after 72 hr since the cultured hepatocytes expressed GS, β-Catenin and HIF-1α , markers of zone-3 hepatocytes. HCV infection also induced phosphorylated-C/EBPβ-Thr266 and proliferation in these cultured hepatocytes. A peptide designed to selectively inhibit phosphorylation of C/EBPβ-Thr266 blocked this phosphorylation, the zone-3 phenotype and hepatocyte proliferation induced by HCV infection in these cultured human hepatocytes. In addition, we found that in normal, uninfected human hepatocytes, unphosphorylated-C/EBPβ-Thr266 was associated with protein von Hippel Lindau (pVHL) and Axin (inhibitors of HIF-1α and β-catenin activation, respectively). These associations were blocked by the HCV infection since phosphorylated C/EBPβ-Thr266 was not associated with either pVHL or Axin. The inhibition of C/EBPβ-Thr266 phosphorylation normalized the association of unphosphorylatable C/EBPβ-Thr266 with pVHL and Axin, which is expected to inhibit the activation of HIF-1α and β−catenin. Collectively, these results strongly suggest that C/EBPβ-Thr266 phosphorylation is indispensable for the activation of GS, HIF-1α and β-catenin, and the induction of zone-3 phenotype in HCV-infected human hepatocytes.
Keywords: Human hepatocytes, Hepatitis C, Liver zone-3, C/EBPβ, Glutamine synthetase, HIF-1, β-catenin
C/EBP, CCAAT/Enhancer Binding Protein; HIF, Hypoxia Inducible Factor; GS, Glutamine Synthetase; HCC, Hepatocellular Carcinoma; pVHL, protein von Hippel Lindau; HCV, Hepatitis C Virus
The hepatocytes in different liver acinar zones are under different Ο2 tension and perform different metabolic activities.1 Hepatocytes in acinar zone-1 are under normoxic conditions, which stimulate aerobic (mitochondrial oxidative) metabolism. By contrast hepatocytes in acinar zone-3 are exposed to hypoxia, which induces survival by hypoxic/‘anaerobic’ (non-mitochondrial) metabolism. Zone-3 hepatocytes are characterized by the expression of Glutamine Synthetase (GS), β-Catenin and Hypoxia-Inducible Factor (HIF)-1.2-5 Expression of the GS gene in zone-3 hepatocytes is stimulated by C/EBPβ.6
We have shown that TGFα induces phosphorylation of mouse C/EBPβ on Thr217 as well as hepatocyte proliferation.7 The C/EBPβ-Thr217 phosphoacceptor is highly conserved through evolution.7,8 We reported that expression of a catalytically inactive mutant RSK, which behaves as a dominant negative, blocks hepatocyte proliferation induced by TGFα, indicating that RSK activity is important for this effect.7
Phosphorylation of C/EBPβ on Thr217 is required for the stimulation of hepatocyte proliferation by TGFα since hepatocytes expressing a C/EBPβ-Ala217 mutant, lacking the critical phosphoacceptor, are refractory to the stimulation of hepatocyte proliferation by TGFα. Also, expression of the phosphorylation-mimic C/EBPβ−Glu217 transgene was sufficient to induce hepatocyte proliferation in the absence of TGFα. In contrast, expression of the C/EBPβ−Ala217 transgene blocked hepatocyte proliferation in the presence of TGFα.7 Thus, C/EBPβ PhosphoThr217 plays an active role in inducing hepatocyte proliferation after treatment with TGFα.
In this study we asked whether Hepatitis C Virus infection in cultured human hepatocytes stimulates C/EBPβ-Thr266 phosphorylation (the human homologue phosphoacceptor) and whether it is required for hepatocyte proliferation and a zone-3 phenotype.
In this study, we assessed the effects HCV infection in the highly-differentiated primary human hepatocyte culture that we have developed for the efficient replication of intact HCV virions. We used primary human hepatocytes (Invitro technologies; Baltimore, MD) cultured on a collagen matrix and infected on day-3 after plating the cells, with serum from a patient with a chronic HCV genotype 1 infection as described previously.9 The matrix was rat-tail collagen( BD Biosciences); the collagen matrix was prepared within 24 hr of hepatocyte plating , at a concentration of 50 mg/ml or greater; the culture plates were coated with polylysine; the suspended hepatocytes were allowed to attach in 20% fetal calf serum for not more than 18 hr; the hepatocyte-specific media was given for at least 24 hr prior to the HCV infection; the hepatocytes were >85% confluent until the time of infection; and hepatocyte media was changed every 72 hr.9
This normal human hepatocyte culture system is permissible to the infection with, and physiologically significant amplification of, naturally occurring HCV.9
We found that HCV genotype 1 infection after 72 hr (viral load ∼ 7 x106 IU/uL) stimulated a zone-3 phenotype since the cultured hepatocytes expressed HIF-1α and GS.2,5 HCV infection also induced phosphorylated-C/EBPβ-Thr266 (the human homologous phosphoacceptor7,8,10) in these cultured hepatocytes. Control uninfected, normal quiescent cultured human hepatocytes did not express phosphorylated-C/EBPβ-Thr266, HIF-1α or GS (Figure 1).

Figure 1 Phosphorylation of human C/EBPβ-Thr266 induced by HCV infection is indispensable for the zone-3 phenotype. Primary human hepatocytes infected with HCV (viral load ~ 7 x106 IU/uL after 72) also express phosphorylation of C/EBPβ-Thr266 and a zone-3 phenotype (HIF-1α; GS). Blocking phosphorylation of C/EBPβ-Thr266 with the dominant negative peptide (100 nM for 24 hr) prevents expression of the zone-3 phenotype. Nuclear stain and the merge images are shown.
We found that HCV infection did not affect the expression of unphosphorylated C/EBPβ-Thr266 compared to control uninfected hepatocytes but stimulated hepatocyte proliferation as determined by the expression of ki67. Hepatocyte proliferation occurred in zone-3 phenotypic hepatocytes, judging by the expression of β-Catenin, another marker of zone-33,4 (Figure 2).

Figure 2 Phosphorylation of human C/EBP-Thr266 induced by HCV infection is indispensable for the expression of -Catenin and hepatocyte proliferation. Experiments were performed as described in Figure 1. Normal or HCV-infected primary human hepatocytes express unphosphorylated C/EBPβ-Thr266. HCV-infected primary human hepatocytes express -Catenin and a marked increase in zone-3 hepatocyte proliferation (ki67 + hepatocytes). Blocking phosphorylation of C/EBPβ-Thr266 with the dominant negative peptide (100 nM for 24 hr in 72-hr HCV-infected human hepatocytes) prevented expression of the zone-3 -Catenin and hepatocyte proliferation. Nuclear stain and merge images are shown.
To test whether phosphorylated-C/EBPβ-Thr266 is required for zone-3 phenotype and hepatocyte proliferation in HCV infection, we treated 72 hr HCV-infected hepatocytes cultures with a peptide designed to selectively inhibit phosphorylation of C/EBPβ-Thr266.8 The peptide blocked this phosphorylation (Figure 1) but did not affect the expression of unphosphorylated C/EBPβ-Thr266 (Figure 2). The peptide also blocked the zone-3 phenotype (expression of HIF-1, GS and β-Catenin as well as hepatocyte proliferation induced by HCV infection (Figures 1 and 2).
In addition, we found that in normal, uninfected human hepatocytes, unphosphorylated-C/EBPβ-Thr266 was associated with protein von Hippel Lindau (pVHL) and Axin (inhibitors of HIF-1α and β-catenin activation, respectively)11,12 (Figure 3).
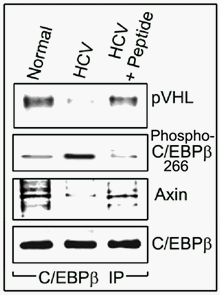
Figure 3 Unphosphorylated-C/EBPβ-Thr217 is physically associated with pVHL and Axin. Experiments were performed as described in Figure 1. C/EBPβ was immunoprecipitated from the 72-hr HCV-infected human hepatocyte’s cell lysates. Unphosphorylated-C/EBPβ-Thr217 was physically associated with pVHL and Axin in uninfected normal primary human hepatocytes (Left lane) and in HCV-infected cultures treated with the inhibitory peptide (Right lane). In HCV-infected human hepatocytes not treated with the peptide, phospho-C/EBPβ-Thr266 is not associated with either pVHL or Axin (Center lane).
These associations were blocked by the HCV infection since phosphorylated C/EBPβ-Thr266, which stimulates HIF-1α and β-catenin activation and expansion of zone-3 phenotype (and the resultant increase in hepatocyte proliferation), was not associated with either pVHL or Axin. The inhibition of C/EBPβ-Thr266 phosphorylation normalized the association of unphosphorylatable C/EBPβ-Thr266 with pVHL and Axin (Figure 3), which is expected to inhibit the activation of HIF-1α and β−catenin (Figure 1 and 2). Further, the interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia.13
Collectively, these results strongly suggest that C/EBPβ-Thr266 phosphorylation is indispensable for the activation of GS, HIF-1 and catenin, and the induction of zone-3 phenotype in HCV-infected human hepatocytes. These novel findings support the activation of catenin and HIF-1 by phospho-C/EBPβ-Thr217 in zone-3 and their inhibition by the peptide blocking the zone-3 phenotype.
We hypothesize that the induction of hepatocyte proliferation confers a survival advantage to the HCV infection. The HCV-induced hepatocyte proliferation is mediated in cultured human hepatocytes by C/EBPβ-Thr266 phosphorylation and the adoption of a zone-3 hepatocyte phenotype.
We thank Daniela Traykova and Caitlin Stalling for their technical support. This study was supported by following grants: RC1 Challenge-DK 087031 (to MB and MC); MERIT R37-DK-46071 (to MC); R01-DK-084139 (to MB), and the Department of Veterans Affairs: Merit Review Award (to MC).
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.