Journal of
eISSN: 2573-2897


Research Article Volume 3 Issue 5
1University of Strasbourg, France
2Avignon Université/CNRS/IRD/AMU, Restoration Engineering of Natural and Cultural Heritage, France
Correspondence: Jacques Connan, 23 rue Saint-Exupéry, 64000-Pau, France
Received: September 12, 2018 | Published: October 15, 2018
Citation: Connan J, Mouton M. Frankincense and bitumen of the middle period (1st century-5th century AD) from the ancient Harbour of Qâni’ (Yemen). J His Arch & Anthropol Sci. 2018;3(5):696-721. DOI: 10.15406/jhaas.2018.03.00153
Thirty-nine samples from the religious complex of Qâni’ which was excavated by the French Archaeological Mission, and two samples from a burnt-out warehouse in Sector VI, which was excavated by the Russian Archaeological Mission in Yemen, were investigated by screening geochemical techniques (Rock-Eval, dichloromethane extract, GC-MS of steranes and terpanes, carbon and deuterium isotopes of asphaltenes) completed by devoted GC-MS analyses in order to identify frankincense. Several types of frankincense (well preserved, thermally altered and carbonized), were identified. In addition sterane and terpane patterns reveal the occurrence of unexpected bitumen in potsherds. Bitumen samples were compared to some oil seeps and crude oils from Yemen but none of the potential sources showed geochemical characteristics matching the bitumen from Qâni’. The occurrence of 18a(H)-oleanane in an ozokerite suggests a possible origin from Khuzistan or Fars in Iran. A specific flowchart, giving access to the predominant triterpenic biomarkers, namely structures with oleanane, ursane and lupane skeletons allowed excluding Boswellia frereana as the frankincense present in the sample set. Various states of thermal degradation of frankincense were found among the frankincense samples of the religious complex through the occurrence of functionalized molecule which were formed by thermal processes.
Keywords: bitumen, frankincense, archaeological samples, crude oils, natural asphalts, gas chromatography-mass spectrometry, Rock-Eval, Yemen, religious complex
The religious complex of Qâni’ was excavated by the French Archaeological Mission in Jawf-Hadramawt in 1995 and 1996,1 and a burnt-out warehouse in the Sector VI, was excavated by the Russian Archaeological Mission in Yemen between 1985 and 1994.2 Thirty-nine samples were gathered during the various campaigns to be investigated by geochemical techniques in order to identify frankincense.1−4 The archaeological site near the modern village of Bi’r’Ali on the coast of the Arabian Sea cost, 100 kms southwest of al-Mukalla, is located in a bay (Figure 1). The site was easily identified as ancient Qâni’ and its name appears in two inscriptions found there, CIH 621 and CIH 728. A Russian team excavated the site between 1972 and 1994. The French Archaeological Mission in Jawf-Hadramawt worked on the site for three seasons between 1995 and 1997 in collaboration with A. Sedov, head of the Russian team. An Italian Mission, directed by B. Davidde, ran two seasons of underwater excavations in the bay of Bi’r’Ali in 1996 and 1998.3 The environment of the site does not lend itself to agriculture and only barely to pastoral activities. The bedrock is basalt covered by sand. Subterranean sweet groundwater is extant and feeds wells that are still in use today. This was a royal foundation in a remote region and dependent on an organized supply system. In founding Qâni’, the intention of the rulers in Shabwa, capital of the Kingdom of Hadramawt, was to participate in the flourishing sea trade, as well as to create a maritime outlet for its own production of resins. Sailors believed, and this is borne out by maritime charts, that Bi’r’Ali was the bay with the best location along the whole Arabian coast between Aden in Yemen and Ra’s Al Hadd in Oman, and the black peak of Husn al-Ghurāb is an excellent navigational landmark.
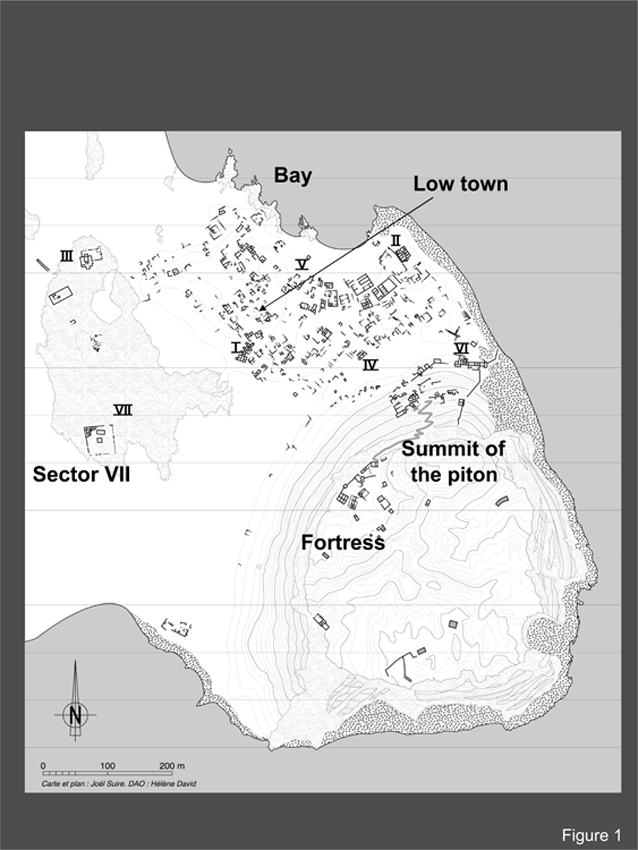
Figure 1 The ancient city of Qâni’ (©Joël Suire and Hélène David/Mission archéologique Jawf-Hadramawt).
Since it was found in 1st century BCE, Qâni’ maintained its position as the main harbour of South Arabia for six centuries. The site’s phase of expansion occurred between the very end of 1st century AD-late 4th or early 5th century AD. Originally a royal warehouse,8 the site gradually evolved into a town with dwellings and warehouses spread along its coast bordering the well-protected bay. To the west of the residential zone, two or perhaps, three sanctuaries were located on a long, flat slab of lava. Qâni’ was at the heart of the aromatic resins trade. Resins were transported to its warehouses either by caravan routes from the interior of the Hadramawt, or by sea from foreign ports, including Sumhurân (Khor Rori), from where the Dhofar’s production was chipped.4 The samples presented here were found in situ in the archaeological layers of two excavated areas of the site.
Sector VII, from where most samples originated, is an enclosed religious complex (40x42m, Figure 2).5 In its central area, the main sanctuary was reached by a straight staircase on a 7.2x7.3 m podium. To the north, an 11-metre-long long hypostyle hall was probably used for ritual meals and in the northwest corner of the enclosure, two rows of rooms were dedicated to the activities of the clergy (cooking equipment, common objects) which were later completed by a small cella which was likely to have sheltered a religious statue. Small stone platforms were built around the central sanctuary and the open space to the south contained approximately fifty hearths that were irregularly distributed in the sand. A warehouse (Sector VI, Figure 2),5 was razed by fire at the end of the 2nd century or the beginning of the 3rd century AD. While working there, the Russian Mission discovered a large quantity of frankincense that was present in coffins6 which were preserved by the fall of the roof. The frankincense is of exceptional quality, according to present-day Yemenites.
Some preliminary observations of the samples, which were carried out by team members familiar with natural fragrances and the different varieties of Yemen’s aromatic mixtures, were sufficiently encouraging to justify undertaking chemical analyses. Incense burners excavated in South Arabia attested to this diversity: they were inscribed with the names of 13 different perfumes7 in South Arabian script. Names on Sabaean incense burners attested to the use of other products such as different varieties of woods, barks, roots, leaves and herbs. They may have been, as they still are, prepared and blended to be sold in souks as burhūr, or added to incense burners during fumigations. The results from resins found in Qanî’ will complete the previously published study that was carried out at Sharma on Middle Age samples that identified copal from Madagascar as the main resin excavated from the warehouse.15 Frankincense was not totally absent but its scarcity (confirmed in only two samples) raised the question of the real relationship of Sharma with the Hadrami hinterland. Some references to black amorphous residues suggest occurrences of burned materials, or mixtures of resin with bitumen, but the lack of analytical proof did not permit confirmation of this hypothesis. Geochemical analyses used for the identification of petroleum were applied as screening techniques to classify the samples and to characterize bitumen when recognized among the samples.16 Specific methods, corresponding to spectroscopic (FT-IR) and chromatographic tools (GC-MS), already applied for the characterization of frankincense, were used on a selection of samples presumed to be composed of this resin.17 In addition, mineralogical analyses were carried out to document the minerals used in mixtures with frankincense and bitumen. These mixtures are still prepared today to be used in fumigations.
Samples of so-called aromatic resins collected during excavations of Sector VII may be separated into four groups according to their geographic location (Table 1):
Data bank number |
Ref number |
Excavation campaign |
Excavation unit |
Sample reference |
Petroleum analyses |
Frankincense analyses |
Macroscopic description |
Location |
Date |
Comment |
961 |
1 |
present-day frankincense. pale yellow and yellow resin, some orange grains |
souk of Sana'a |
pure frankincense |
||||||
962 |
2 |
black sample, hard with vacuolar texture. Burned? |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
on a table for frankincense |
|||||
963 |
3 |
numerous black debris with vacuolar texture. Burned? |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
at the foot of the temple staircase |
|||||
964 |
4 |
white powder |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
on a table for frankincense |
|||||
1257 |
5 |
QAN.95 |
UF28 |
e.77 |
yellow powder with centimetric clusters |
end of AD 1st century to beginning of AD 5th century |
outside P51 (habitat or administration of priests) |
|||
1258 |
6 |
QAN.95 |
UF35 |
e.118 |
yellow powder without any scent |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level |
||
1259 |
7 |
QAN.95 |
UF.6 |
e.18 |
yellow powder without marked scent.some orange grains |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
surface |
||
1260 |
8 |
QAN.95 |
UF15 |
e.93 |
black powder.Gray-brown mixture with vacuolar structures and some well-preserved grains. Partly burned? |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level |
||
1261 |
9 |
QAN.95 |
UF31 |
e.104 |
brown mass with mineral elements and vacuolar structures. Burned? |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level, frankincense collected at the NE angle of the stair, on an incense burner |
||
1262 |
10 |
QAN.95 |
UF15 |
e.95 |
brown mass, heterogenous, carbonised, vacuolar structures |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level, ashes on a burned sanstone plate |
||
1263 |
11 |
QAN.95 |
UF31 |
e.103 |
black powder, glossy, highly carbonised? |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level |
||
1264 |
12 |
QAN.95 |
UF31 |
e.120 |
brown mass with vacuolar structures. Carbonised? Some sea shel land minerals trapped in the lump |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level |
||
1265 |
13 |
QAN.95 |
UF26 |
e.105 |
brown mass inside a potsherd. Vacuolar structures of degasing but no evidence of burning |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level, potsherd |
||
1266 |
14 |
QAN.95 |
UF17 |
e.65 |
white powder agglomerated in 0.5 cm balls |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level |
||
1267 |
15 |
QAN.95 |
UF29 |
e.98 |
white powder |
central sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
occupation level |
||
1268 |
16 |
QAN.95 |
UF5 |
e.34 |
1268a- yellow-reddish resin. 1268b-white-yellow powder |
end of AD 1st century to beginning of AD 5th century |
surface |
|||
1269 |
17 |
QAN.96 |
UF38 |
e.139 |
pure solid bitumen with chonchoidal structure |
In hearths, at the beginning of the pathway leading to the sanctuary P59 |
end of AD 1st century to beginning of AD 5th century |
|||
1270 |
18 |
QAN.96 |
UF39 |
e.183 |
yellow to orange grains- resin with a balsamic scent |
in the destruction of altars (?) P107 and P92 |
end of AD 1st century to beginning of AD 5th century |
|||
1271 |
19 |
QAN.96 |
UF59 |
e.220 |
pale yellow to white powder , red-orange grains |
occupation of P10 |
end of AD 1st century to beginning of AD 5th century |
|||
1272 |
20 |
QAN.96 |
UF46 |
e.169 |
orange to red grains |
in the destruction of altars (?) P107 and P92 |
end of AD 1st century to beginning of AD 5th century |
|||
1273 |
21 |
QAN.96 |
UF40 |
e.148 |
gray powder: ashes? |
outdoor space, north of the hall of columns |
end of AD 1st century to beginning of AD 5th century |
|||
1274 |
22 |
QAN.96 |
UF51 |
e.206 |
yellow powder |
in hearths, established in the whole outdoor space, at South-West of the enclosed space |
end of AD 1st century to beginning of AD 5th century |
|||
1275 |
23 |
QAN.96 |
UF40 |
e.146 |
1275a- brown-black lumps. Burned? 1275b black powder, glossy with some sea shells, sometime broken |
outdoor space, north of the hall of columns |
end of AD 1st century to beginning of AD 5th century |
|||
1276 |
24 |
QAN.96 |
UF40 |
e.144 |
yellow powder |
outdoor space, north of the hall of columns |
end of AD 1st century to beginning of AD 5th century |
|||
1277 |
25 |
QAN.96 |
UF58 |
e.196 |
black-brown lumps of several cm, hard and heavy |
surface |
end of AD 1st century to beginning of AD 5th century |
|||
1278 |
26 |
QAN.96 |
UF55 |
e.194 |
some yellow grains |
hall of columns P9, on the upper ground |
end of AD 1st century to beginning of AD 5th century |
|||
1279 |
27 |
QAN.96 |
UF46 |
e.192 |
brown mass with vacuolar structures but not carbonised inside a potsherd. Abundant degassing. |
in the destruction of altars (?) P107 and P92 at NE |
end of AD 1st century to beginning of AD 5th century |
|||
1280 |
28 |
QAN.96 |
UF58 |
e.204 |
white powder in small grains, homogeous and pure |
surface |
end of AD 1st century to beginning of AD 5th century |
|||
1281 |
29 |
QAN.96 |
UF64 |
e.240 |
brown mass with a carbonised aspect but likely amixture of bitumen and minerals |
on the ground of the pillared hall P9 |
end of AD 1st century to beginning of AD 5th century |
|||
1282 |
30 |
QAN.96 |
UF56 |
e.188 |
Brown to black mass with vacuolar aspect . Burned? |
Hall of columns P9, on the upper ground |
end of AD 1st century to beginning of AD 5th century |
|||
1283 |
31 |
QAN.96 |
UF52 |
e.159 |
yellow to brown resin |
outdoor space, north of the hall of columns |
end of AD 1st century to beginning of AD 5th century |
|||
1284 |
32 |
QAN.96 |
UF48 |
e.164 |
1284a- yellow powder mixed with black powder (burned?).1284b-Black mass (burned?) |
in the destruction of altars (?) P107 and P92 |
end of AD 1st century to beginning of AD 5th century |
|||
1285 |
33 |
QAN.96 |
UF51 |
e.205 |
in hearths, established in the whole outdoor space, at South-West of the enclosed space |
end of AD 1st century to beginning of AD 5th century |
||||
1286 |
34 |
QAN.96 |
secteur VI |
large plate 3-4 cm long and 1 cm thick. White and red-orange grains agglutinated in all directions- pure frankincense? |
sector VI. Burned warehouse excavated by A.V.Sedov |
AD 150-200 or AD 225 |
||||
1287 |
35 |
QAN.96 |
UF36 |
e.140 |
1287a-white powder with mineral grians and black grains (burned resin?). 1287b-brown-black mass with vacuolar structures |
surface and occupation |
end of AD 1st century to beginning of AD 5th century |
At the foot of altars, bordering the driveway leading to the sanctuary P59 |
||
1288 |
36 |
QAN.96 |
UF58 |
e.207 |
red-orange resin |
surface |
end of AD 1st century to beginning of AD 5th century |
|||
1289 |
37 |
QAN.96 |
UF75 |
e.239 |
1289a- carbonised wood? 1289b brown mass with vacuolar structures (burned?) |
remblais de P.10-reconstruction |
end of AD 1st century to beginning of AD 5th century |
|||
1290 |
38 |
QAN.96 |
UF58 |
e.210 |
black lumps , fairly hard with few vacuoles |
surface |
end of AD 1st century to beginning of AD 5th century |
|||
1291 |
39 |
QAN.96 |
UF36 |
e.143 |
powder with big lump of yellow to orange resin. |
surface et occupation |
end of AD 1st century to beginning of AD 5th century |
At the foot of altars, bordering the driveway leading to the sanctuary P59 |
||
1292 |
40 |
QAN.96? |
secteur VI |
brown mass with vacuolar structures: black glossy powder |
sector VI. Burned warehouse excavated by A.V.Sedov |
AD 150-200 or AD 225 |
||||
1293 |
41 |
QAN.95 |
UF5 |
e.28 |
fragrant substance encountered in balms of Egyptian mummies |
surface |
end of AD 1st century to beginning of AD 5th century |
Table 1 Basic information concerning samples analyzed
Group 1: in relation to sanctuary and altars
Around the large northern altar (18, 20, 21, 23, 24, 27, 31, 32)
Around the central podium (2, 3, 4, 6, 8, 9, 10, 11, 12, 13, 15)
Around the southern altars (19, 28, 36, 38)
Seven samples were collected around the main podium, four on stones supports, which were thought to be at the top of the staircase were they were burned. The path leading to the sanctuary was bordered by three stone altars on each side with another four altars located behind the sanctuary. Several samples were gathered around the eastern facilities and in the ballast from the altars, and these indicated offerings and fumigations.
Group 2: in the hypostyle hall (26, 29, 30).
This hall is interpreted as a refectory or a room used for ritual meals. Fish and mammal bones were found in the occupation level which partly validates this interpretation. The occurrence of a room for meals is attested elsewhere in Hadramawt in association with sanctuaries and were located within the cultural enclosure or integrated in a complex building at Raybûn.12
Group 3: in relation to the northwest corner of the building
Before the building (19)
Within the occupation levels (7, 14, 37)
The worship sets are currently enclosed by a wall and integrated buildings that were used by the clergy for their activities. The enclosure, with the two first western rooms, was built after the construction of the sanctuary and sample 19 corresponds to the utilization of the sanctuary before the building of the enclosure. The remnants of resin discovered in this area may be related to the cella, also housed in the building, where an incense burner was also preserved.
Group 4: in relation to hearths (17, 22, 33, 35? 39?)
Hearths, housed in soft sand, corresponded to ritual practices relating to the sacredness of the place but it was not possible to determine whether they were constructed during the main occupation of the sanctuary or afterwards, when it was partly abandoned. Flat shells (scallop or Pecten maximus) with ash and traces of combustion that may have been used to burn aromatic resins for offerings or during communal meals were found in these hearths.
Two samples from the burnt-out warehouse in Sector VII, excavated by the Russian Archaeological Mission (34, 40) should be added to this group. Macroscopic inspection of these show a diversified panel between white powder (14 and 41, Figure 3) which was thought to be resin mixed with mineral matter (flour of calcite, gypsum): yellow-to-orange organic materials which was undoubtedly resin (18, Figure 3): brown residue which was possibly burned resin (25, Figure 3) or something else (27, Figure 3) and pitch-dark carbonized matter (40, Figure 3). For comparison purposes, a sample of present-day frankincense (1) from the market at Sana’a was also incorporated into the sample set.

Figure 3 The different macroscopic aspects of samples analyzed.
No.14#1266: sample containing calcium sulphate. No.41 #1293: sample with calcite, gypsum and NaCl. No. 18 #1270: preserved frankincense. No 25 #1277: thermally altered frankincense. No 27 # 1279: bitumen inside a potsherd. No.40 #1292: “carbonized” organic matter.
Rock-eval pyrolysis
The Rock-Eval pyrolysis18 which is widely used by the petroleum industry to characterize source rocks, has been adapted as a screening technique for archaeological samples (pitch, bitumen, resins, tar, etc.).19,20 A small amount of raw sample (100 mg) was heated under helium at 300°C for three minutes and then the temperature was increased up to 600°C at 25°C/min. The thermovaporization at approximately 300°C gave a first peak (S1) corresponding to free hydrocarbons and is expressed in mg HC/g of sample. Hydrocarbons and CO2 formed by cracking between 300 and 600°C gave the second peak (S2) which is also expressed in mg HC/g sample, and a third peak (S3) refers to oxygen-bearing compounds transformed into CO2 in mg CO2/g sample. The temperature of the maximum of the S2 peak (Tmax) is expressed in °C. The organic carbon remaining after pyrolysis was obtained by oxidation under air (or oxygen) atmosphere at 600°C, and the resulting CO2 peak (S4) is expressed in mg CO2/g sample. The total organic carbon (TOC in weight %) is computed using S1, S2 and S4.
Reagents and solvents
All solvents and reagents were of analytical grade from Meck (Darmstadt, Germany). Crystalline reference samples of all triterpenic standards from frankincense were isolated and characterized in Laboratoire de Chimie Bioorganique.21 a- and b-amyrins and lupeol were purchased from Extrasynthese (Genay, France) and b-amyrenone and lupenone were obtained from b-amyrin and lupeol by oxidation with pyridinium chlorochromate (PCC) in dichloromethane. Derivatization reagents employed to treat samples were hexamethyldisilazane (HMDS) and chlorotrimethylsilane (TMSCI), and the solvents used were pyridine (Sigma-Aldrich, ACS reagent, >99.0%) and dichloromethane (Sigma-Aldrich, ACS reagent, >99.5%).
Chromatographic analyses were carried out using a Varian Saturn 3900 gas chromatograph, with a Varian 1177 injector, coupled to a Varian 2100 T ion-trap mass spectrometer. Separation was performed on a capillary column Varian CP-Sil 5 CB Low Bleed/MS, 30 mx0.25 mm i.d.x0.25 µm. The carrier gas was helium at a constant flow of 1mL/ min-1. The GC injector temperature was 250°C. The initial oven temperature was 50°C maintained for two mins during splitless injection. The injection volume was 1µL. A 8°C.min-1 gradient was then applied to the oven until 250°C was attained, and another 3°C.min-1 gradient was applied until 350°C was reached. No hold time was performed at the upper limit. Ionization was carried out with standard conditions at 70 eV. The mass spectrometer was scanned in the m/z 50-650 range, with a cycle time of one second.
Derivatization procedure
Five mg of the material was trimethylsilylated with a solution of pyridine (0.5 mL), HMDS (0.45 mL) and TMSCI (0.3 mL). This derivatization reaction occurred at room temperature (30 mins) before the solution was dried with a stream of nitrogen while heating (<40 °C). Thereafter, the residue was dissolved in 0.6 mL of dichloromethane, and after filtration on PTFE cartridge, it was directly injected into the gas chromatographic system.
FT-IR spectroscopy
All infrared spectra were collected at room temperature with a Nicolet (Madison, Wisconsin, USA) Avatar 360 ASP spectrometer equipped with a DTGS KBr detector and controlled by EZ OMNIC 60 software. The IR spectra were collected with 64 scans and 4 cm-1 resolution. Samples were homogeneously crushed with anhydrous potassium bromide in a proportion of 1/20. The powder was then compressed under 10t/cm2 to form translucent pellets.
Analysis of the extractable organic matter
The soluble portion of the sample was extracted with dichloromethane, and the emphasis was on the analysis of “saturated” hydrocarbon fractions. The dichloromethane extract was fractionated into three classes of compounds (“saturated hydrocarbons”, “aromatic hydrocarbons” and polar compounds) by IATROSCAN MK IV apparatus on Chromarods (5 µ silica SIII). The quantitative measurement of each fraction was carried out by a flame ionization detector to obtain a gross composition of the extract. After the precipitation of asphaltenes by n-hexane and their quantitative gravimetric measurement, an aliquot of deasphalted extract (100 mg) was fractionated by MPLC (medium pressure liquid chromatography) on a LOBAR MERCK column (300 mm x10 mm, Lichro-prep 30-60 µm), activated at 120°C under N2 for 30 mins. The “saturated hydrocarbon” fraction was eluted with n-hexane. Recovery of the “aromatic hydrocarbon” fraction was achieved with n-hexane using a back-flush step on both columns (pre-column and analytical column LOBAR-MERCK). This step could have re-introduced some polar compounds (ketones, esters) into the so-called “aromatic hydrocarbon” fraction in the case of archaeological samples.19 The two “hydrocarbon” fractions in the asphalts were mainly C15+alkanes and C15+aromatics. Finally, the polar compounds were eluted with dichloromethane/methanol (85/15) in back-flush mode from the pre-column. Each recovered fraction was then weighed.
Gas chromatography-Mass spectrometry (GC-MS) analysis of “saturated hydrocarbon” was performed using a Hewlett-Packard 6890/5973 GC-MS instrument equipped with an on-column injector and operating at 70 eV with a mass range of m/z 50-550. A DB5 fused silica capillary column was used (J&W, 60 mx0.25 mm, film thickness 0.1 µm). The samples were injected at 75°C, and the oven was programmed at 2°C/min to 300°C, where the temperature was held for 66 mins. d13C were measured by an on-line process that couples a Carlo-Erba 2500 elemental analyzer with a Finnigan Delta Plus mass spectrometer via a Conflo II unit. The sample was transported by a helium flow (15 psi, 86 ml/min) and oxidized by pure oxygen (17.5 psi, 37 ml/min) at a temperature of 1020°C in a furnace containing tungsten oxide on alumina. An on-line magnesium perchlorate trap was used to remove the water formed by combustion. The generated CO2 was introduced into the mass spectrometer. A pulse of reference CO2 with known d13C was injected before the CO2 samples peak. The NBS 127 standard was used to calibrate the instrument and control the whole methodology. Isotope values are provided with an uncertainty of ±0.2‰/VPDB for CO2.
dD was measured by an off-line process. The sample was oxidized with copper oxide at 590°C for 15 hours. The water produced with copper oxide at 590°C for 15 hours. The water produced by the oxidation was subsequently reduced with zinc at 470°C for 30 mins and the resultant hydrogen introduced via a dual inlet into a Finnigan Delta Plus mass spectrometer. A known standard was used to calculate the D value of the sample. The NBS 22 standard was used to calibrate the instrument. Deuterium values are given with an uncertainty of ± 4‰/SMOW.
Rock-Eval screening analysis
Rock-Eval pyrolysis on whole samples provided access to total organic carbon (TOC in %/sample) and, therefore, evaluated the contribution of organic matter in the samples. Other pyrolytic parameters, deduced from the pyrograms that are shown for four samples in Figure 4, provided the Hydrogen Index (HI in mg HC/g TOC), the Oxygen Index (OI in mg CO2/g TOC) and the Tmax (in °C) of the S2 peak, and gave some insight into the nature of the sample. The plot of the Hydrogen Index as a function of Total Organic Carbon (TOC in w. % /sample) in Figure 5 showed a wide variety of sample properties: TOC varies from 0.18% -73% when HI ranged from 39-746 mg HC/g TOC. These data suggested that the sample set contains some preserved raw resins (e.g. samples 15 and 22) but also burned or highly carbonized samples (e.g. samples 11 and 30) and even some residual ashes (sample 21, not shown). In addition, the samples may contain rich in organic matter as indicated by TOC higher than 50% but were also composed of organic matter diluted by mineral phases, which may even be dominant. The nature of this mineral phase was explored on some samples by using X-ray diffraction analysis and FTIR analysis. The plot of the Hydrogen Index as a function of Tmax and OI in figure 6a confirmed the diversity of the encountered situation and a tendency of increasing the Tmax when the HI was reduced. This property fits with the occurrence of resins that are both well preserved and burnt. The choice of a representative Tmax may be sometimes difficult to assess due to flat (sample 5, Figure 4) or bimodal curves (samples 5 and 17). However, subsequent molecular information clarified the raw parameters, enabling the two main groups of samples in the whole set to be distinguished: those that contained bitumen and those in which bitumen was lacking. Bitumen-bearing samples are characterized by lower Oxygen Index and higher Tmax (Figure 6a & Figure 6b). A Tmax between 420°C and 432°C is currently seen in archaeological samples.20,21 Sample 13, which is bitumen-bearing, displayed a typical bitumen value at 434°C (Figure 4). The detailed molecular analysis shown below provides some insight into observed differences among bituminous samples by indicating that Sample 17 is pure paraffinic bitumen called ozokerite. This specific character explains the bimodal pyrogram with the main peak related to paraffin contribution at 392 °C and the shoulder at 438 °C to polymeric macromolecules (Table 2).
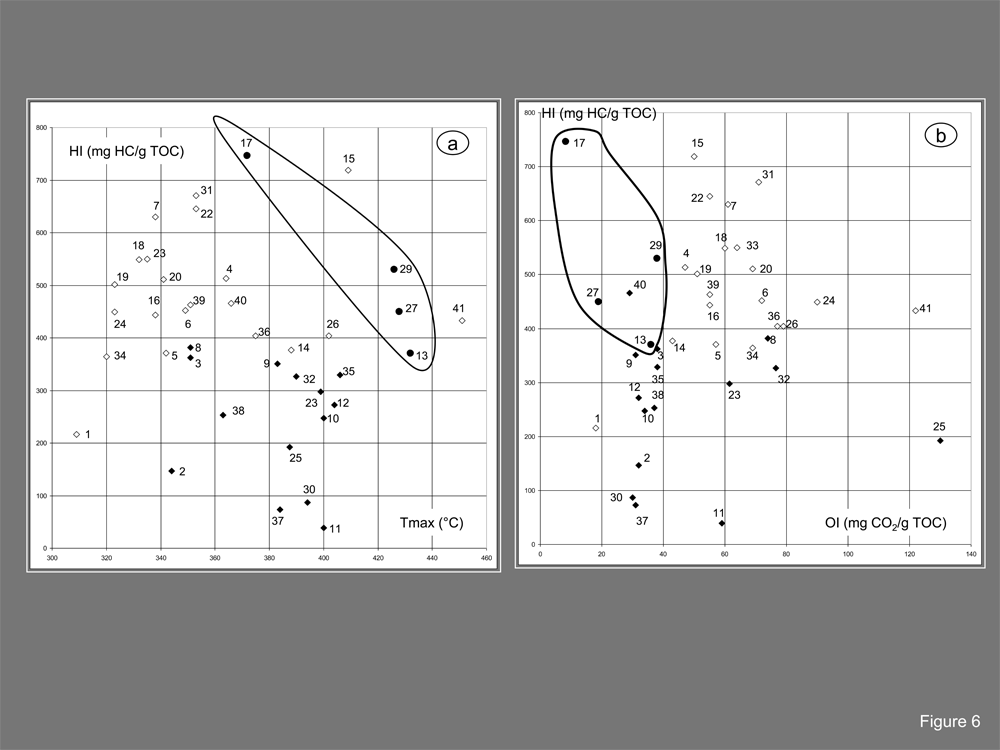
Figure 6 Rock-Eval data: (A) plot of HI (mg HC/g TOC) vs. Tmax (°C). (B) plot of HI (mg HC/g TOC) vs. OI (mg CO2/g TOC).
Data bank number |
Ref number |
Origin of sample |
Tmax |
S1 |
S2 |
S3 |
TOC |
IP |
OI |
H1 |
961 |
1 |
Present-day frankincense. pale yellow and yellow resin, some orange grains |
309 |
627 |
142 |
12.2 |
65.5 |
0.81 |
18 |
216 |
962 |
2 |
Black sample, hard with vacuolar texture. Burned? |
344 |
52.4 |
48.7 |
10.7 |
33.1 |
0.52 |
32 |
147 |
963 |
3 |
Numerous black debris with vacuolar texture. Burned? |
351 |
87.1 |
78.3 |
8.3 |
21.6 |
0.53 |
38 |
362 |
964 |
4 |
white powder |
364 |
114.7 |
105.1 |
9.8 |
20.5 |
0.52 |
47 |
513 |
1257 |
5 |
yellow powder with centimetric clusters |
342 |
53.6 |
25.8 |
4 |
6.9 |
0.68 |
57 |
371 |
1258 |
6 |
yellow powder without any scent |
349 |
396 |
279.8 |
45.1 |
61.8 |
0.58 |
72 |
452 |
1259 |
7 |
yellow powder without marked scent.some orange grains |
338 |
255.4 |
360.6 |
35.2 |
57.2 |
0.41 |
61 |
630 |
1260 |
8 |
black powder.Gray-brown mixture with vacuolar structures and some well-preserved grains. Partly burned? |
351 |
128 |
94.8 |
18.6 |
24.8 |
0.57 |
74 |
382 |
1261 |
9 |
brown mass with mineral elements and vacuolar structures. Burned? |
383 |
81.9 |
85.9 |
7.7 |
24.6 |
0.48 |
31 |
351 |
1262 |
10 |
brown mass, heterogenous, carbonised, vacuolar structures |
400 |
104.7 |
62.8 |
8.8 |
25.3 |
0.63 |
34 |
247 |
1263 |
11 |
black powder, glossy, highly carbonised? |
400 |
26 |
13.8 |
20.8 |
36.3 |
0.65 |
59 |
39 |
1264 |
12 |
brown mass with vacuolar structures. Carbonised? Some sea shel land minerals trapped in the lump |
404 |
92.8 |
81.6 |
9.8 |
30 |
0.53 |
32 |
272 |
1265 |
13 |
brown mass inside a potsherd. Vacuolar structures of degasing but no evidence of burning |
432 |
12.1 |
31 |
3 |
8.3 |
0.28 |
36 |
370 |
1266 |
14 |
white powder agglomerated in 0.5 cm balls |
388 |
29.48 |
13.44 |
1.55 |
3.56 |
0.69 |
43 |
377 |
1267 |
15 |
white powder |
409 |
133 |
319.6 |
22.3 |
44.4 |
0.29 |
50 |
719 |
1268a |
16 |
1268a- yellow-reddish resin. 1268b-white-yellow powder |
338 |
354.5 |
247.1 |
31.3 |
55.7 |
0.59 |
55 |
443 |
1269 |
17 |
pure solid bitumen with chonchoidal structure |
372 |
293.4 |
546 |
6.1 |
73 |
0.34 |
8.3 |
746 |
1270 |
18 |
yellow to orange grains- resin with a balsamic scent |
332 |
283.4 |
307.7 |
33.9 |
56 |
0.48 |
60 |
549 |
1271 |
19 |
pale yellow to white powder , red-orange grains |
323 |
274.8 |
247.7 |
25.2 |
49.4 |
0.53 |
51 |
501 |
1272 |
20 |
orange to red grains |
341 |
286.1 |
296.1 |
40.3 |
57.8 |
0.49 |
69 |
511 |
1274 |
22 |
yellow powder |
353 |
51.6 |
73.4 |
6.3 |
26.2 |
0.41 |
55 |
645 |
1275 |
23 |
1275a- brown-black lumps. Burned? 1275b black powder, glossy with some sea shells, sometime broken |
399 |
59.7 |
45.4 |
9.4 |
15.9 |
0.57 |
62 |
298 |
1276A |
24 |
yellow powder |
323 |
203.1 |
150.5 |
30.4 |
33.5 |
0.57 |
90 |
449 |
1277 |
25 |
black-brown lumps of several cm, hard and heavy |
388 |
45.8 |
19.3 |
12.1 |
9.9 |
0.7 |
130 |
192 |
1278A |
26 |
some yellow grains |
402 |
143.4 |
91.4 |
18 |
22.6 |
0.61 |
79 |
404 |
1279 |
27 |
brown mass with vacuolar structures but not carbonised inside a potsherd. Abundant degassing. |
428 |
27.7 |
88.1 |
3.8 |
19.5 |
0.24 |
19 |
449 |
1281 |
29 |
brown mass with a carbonised aspect but likely amixture of bitumen and minerals |
426 |
12.9 |
89.1 |
6.3 |
16.8 |
0.12 |
38 |
529 |
1282 |
30 |
Brown to black mass with vacuolar aspect . Burned? |
394 |
44.4 |
33.6 |
11.8 |
38.6 |
0.57 |
30 |
87 |
1283 |
31 |
yellow to brown resin |
353 |
36.9 |
62.2 |
6.6 |
9.3 |
0.37 |
71 |
671 |
1284 |
32 |
1284a- yellow powder mixed with black powder (burned?).1284b-Black mass (burned?) |
390 |
112.1 |
57.3 |
12.5 |
18.3 |
0.66 |
77 |
327 |
1285 |
33 |
335 |
304 |
328.3 |
38.6 |
59.6 |
0.48 |
64 |
550 |
|
1286 |
34 |
large plate 3-4 cm long and 1 cm thick. White and red-orange grains agglutinated in all directions- pure frankincense? |
320 |
257.9 |
134.9 |
25.6 |
37 |
0.66 |
69 |
364 |
1287A |
35 |
1287a-white powder with mineral grians and black grains (burned resin?). 1287b-brown-black mass with vacuolar structures |
406 |
55.2 |
36.8 |
4.2 |
11.2 |
0.6 |
38 |
329 |
1288 |
36 |
red-orange resin |
375 |
354.9 |
217.3 |
41.7 |
53.8 |
0.62 |
77 |
404 |
1289b |
37 |
1289a- carbonised wood? 1289b brown mass with vacuolar structures (burned?) |
384 |
53.3 |
23.5 |
10 |
32 |
0.69 |
31 |
73 |
1290 |
38 |
black lumps , fairly hard with few vacuoles |
363 |
80.5 |
44 |
6.5 |
17.3 |
0.65 |
37 |
253 |
1291 |
39 |
powder with big lump of yellow to orange resin. |
351 |
166.8 |
134.3 |
16 |
29 |
0.55 |
55 |
463 |
1292 |
40 |
brown mass with vacuolar structures: black glossy powder |
366 |
109.6 |
132.7 |
8.4 |
28.5 |
0.45 |
29 |
466 |
1293A |
41 |
fragrant substance encountered in balms of Egyptian mummies |
451 |
0.03 |
0.78 |
0.22 |
0.18 |
0.04 |
122 |
433 |
Table 2 Rock-Eval data of samples analyzed. Significance of abbreviations: S1 (mg HC/g sample), S2 (mg HC/g sample), S3 (mg CO2/g sample), TOC (Total Organic Carbon in %/sample), IP = S1/S1 + S2, OI =Oxygen Index (mg CO2 / g of TOC), HI = hydrogen index (mg HC/g of TOC)
Analysis of the mineral phase
Investigation of the mineral content of the samples was carried out by X-ray diffraction, Fourier transform infrared spectroscopy (FT-IR) and also chemical means on both extracted residues after dichloromethane treatment and whole sample. The results obtained are compiled in Table 3. The selected samples covered a wide range from organic-rich powder (e.g. Samples 6 and 7) to those that were mineral dominant ones (e.g. Samples 28 and 41). The three samples (13, 27 and 29) that were identified as bituminous-bearing samples, were preferentially examined to be incorporated into a wider research program dealing with archaeological bitumen in the Near East and the Gulf.23 Insoluble organic residues were mainly composed of mineral matter (I.O.C. = Insoluble Organic carbon between 0.3% and 3.5 %) with calcite and clays as dominant constituents. Quartz was generally low, except in Sample 34, where it reached 28% with 25% NaCl! Occurrence of aragonite brought by carbonated bioclasts and of NaCl related to sea proximity, favoured a local contribution of bioclastic sands7 However the identification of gypsum (and even anhydrite in one sample) in both extracted and whole sample suggested another source that has yet to be located. Gypsum, found as a major constituent in the white powder of Sample 14 and as crystals with NaCl in Sample 41, was possibly added to carbonated mixtures available locally or from another source located elsewhere.
Insoluble residue after extraction with dichloromethane |
Rock-Eval data on residue |
whole sample |
comments |
|||||||||||||||||||||
Sample number |
sample number |
TOC |
quartz (%) |
calcite (%) |
dolomite (%) |
halite (%) |
aragonite (%) |
gypsum (%) |
anhydrite (%) |
plagioclases (%) |
feldspaths K (%) |
pyrite (%) |
clays&mica (%) |
I.O.C. (%) |
I.R. H2O 15% |
I.R. HCl 15% |
I.O.C. (%) |
HI |
OI |
Tmax |
calcite |
sulfate |
halite |
|
1258 |
6 |
61.8 |
x |
tellow powder without any smell |
||||||||||||||||||||
1259 |
7 |
57.2 |
yellow powder with a slight aromatic smell |
|||||||||||||||||||||
1261 |
9 |
25.5 |
9 |
42 |
3 |
6 |
traces |
0 |
0 |
5 |
0 |
0 |
31 |
3.45 |
93.3 |
48.8 |
3.43 |
77 |
80 |
432 |
powder with white grains (minerals?) + burned frankincense |
|||
1263 |
11 |
36.3 |
x |
x |
black shiny powder |
|||||||||||||||||||
1264 |
12 |
30 |
x |
brown mass , some shells in the mixture |
||||||||||||||||||||
1265 |
13 |
6.5 |
7 |
65 |
3 |
5 |
3 |
0 |
0 |
1 |
0 |
0.2 |
13 |
2.59 |
94.1 |
24.1 |
2.69 |
205 |
60 |
433 |
brown mass with vacuolar figures, some shells |
|||
1266 |
14 |
3.56 |
x |
white powder |
||||||||||||||||||||
1279 |
27 |
18 |
9 |
43 |
5 |
5 |
0 |
4 |
0 |
4 |
0 |
0.4 |
26 |
3.2 |
89.1 |
43.2 |
1.31 |
198 |
86 |
433 |
brown mass which does not seem to be carbonized |
|||
1280 |
28 |
0.09 |
xxx |
gypsum |
x |
white powder. TOC = 0.09%/sample |
||||||||||||||||||
1281 |
29 |
17.8 |
3 |
73 |
4 |
6 |
0 |
0 |
0 |
1 |
0 |
0.2 |
11 |
1.28 |
92.5 |
16.8 |
3.55 |
266 |
52 |
430 |
mass with a carbonized aspect |
|||
1283 |
31 |
9.3 |
x |
x |
yellow to brown resin-TOC = 9.3 %/sample |
|||||||||||||||||||
1286 |
34 |
37 |
28 |
25 |
4 |
25 |
3 |
4 |
3 |
2 |
traces |
0 |
5 |
0.54 |
67.4 |
35.9 |
0.32 |
1034 |
1234 |
430 |
big plate |
|||
1287 |
35 |
11.2 |
x |
x |
white powder |
|||||||||||||||||||
1293 |
41 |
0.18 |
oui |
xxx |
gypsum |
white cristals -gypsum and calcite - TOC = 0.18% |
||||||||||||||||||
Table 3 Mineral composition of some samples
Four archaeological samples (#1258, #1263, #1264 and #1283) contained mineral fraction characterized by FT-IR. The interpretation of the obtained spectra showed that these contain inorganic sulphate and/or carbonate groups (Figure 7). Indeed, the infrared absorption spectra of inorganic carbonate and sulfate compounds were very distinctive. Normal inorganic carbonate linkages presented strong characteristic absorptions that could be observed around 1450-1410 cm-1, 880-860 cm-1 and 750-700 cm-1. The last two lower frequency bands were very sharp. The last was not systematically observed but it is often useful to identify minerals such as calcite and dolomite. In calcium carbonate, these absorption bands occurred at 1430 cm-1 and 876 cm-1. For inorganic sulfates, there were two characteristic bands, the first one, which was the strongest and was relatively broad, was shown in the range 1130-1080 cm-1 while the second one at around 680-610 cm-1, was weaker and very narrow. In calcium sulfate the very strong band was at 1140cm-1.24 We can conclude that samples #1263 and #1264 contain inorganic carbonate compounds corresponding to calcium carbonate and materials #1258, #1263 and #1283 show the presence of calcium sulphate. In these last samples, the simultaneous occurrence of triterpenoids, fatty substances and mineral matter could be a probable result of an anthropic mixture corresponding to a real formulation.
Figure 7 Infrared absorption spectra of four samples showing calcium carbonate in sample #1263 and #1264 and calcium sulphate in samples #1258, #1263 and #1283. Calcium carbonate displays absorption bands at 1430cm-1 and 876cm-1 and calcium sulphate at 1130-108 cm-1 and around 680-610cm-1.
Extractable organic matter
Extractable organic matter (EOM) was isolated from a selection of diversified samples including yellow-to-orange resin-like powder and brown-to-dark brown mixtures, which were free of any support or stuck on potsherds. The quantified data are listed in Table 4. The GC-MS analysis of their respective alkane fractions has enabled us to distinguish samples corresponding to pure, well-preserved frankincense, burned frankincense and bitumen (Figure 8). The discovery of bitumen, not expected when the study was undertaken, entailed a geochemical analysis of the oil seeps in Yemen which outcrop at Balhaf (Wadi Harubya, Figure 9) in the vicinity of Qâni’, from two salt mines at Shabwa (Shabwa 1 and 2, Figure 9),25 and from a tar sand in the neighborhood of the Mintaq 1 well (Figure 10). A plot of the gross composition of extracts in a ternary diagram (% saturates + aromatics vs. % resins, vs. % asphaltenes, Figure 11) shows four differentiated groups corresponding to preserved frankincense, burned frankincense, archaeological bitumen and finally, oil seeps. As usual, oil seeps appear as the most enriched in hydrocarbons, namely “saturates” + “aromatics”, whereas the bitumen of Qâni’ falls within the current area of archaeological oxidized bitumen with an abundant amount of asphaltenes, except for the sample #1269, which clearly belongs to the oil seep family. Its high organic carbon (TOC=72.8 w. %/sample) combined with its exceptional richness in high molecular weight n-alkanes and its distribution peaking at n-C37 refine the diagnostic and indicate that the sample may be classified among geological paraffinic residues called ozokerites.26,27
well |
Geomark or lab number |
lat |
long |
depth (in feet) |
age |
δ13C15+sat |
δ13C15+aro |
δ13Casp |
δ13Cres |
δDasp |
prist/phyt |
C30hopane (ppm) |
%C27 |
%C28 |
%C29 |
Ts/Tm |
C29Ts/Tm |
Olean/H |
GA/C31R |
Rearr/Reg |
Ster/Terp |
C29/H |
S1/S15 |
29Ts |
comment |
Sunah 2 |
XYN0001 |
15.8 |
49.049 |
9039 |
U.Jurassic shales |
-29 |
-27.6 |
1.8 |
403 |
33.5 |
27.7 |
38.8 |
1.59 |
0.89 |
0 |
0.12 |
3.09 |
0.46 |
0.36 |
3.02 |
29Ts |
abundant diasteranes |
|||
Ayad |
YN0001 |
14.9 |
46.865 |
3125 |
M.Jurassic |
-28.5 |
-27 |
1.46 |
289 |
38.4 |
26.7 |
34.9 |
2.01 |
0.63 |
0.01 |
0.04 |
3.42 |
0.52 |
0.35 |
3.96 |
29Ts |
abundant diasteranes |
|||
Alif |
YN0002 |
15.6 |
45.803 |
5700 |
Turonian |
-28.2 |
-26.1 |
1.55 |
761 |
37.3 |
25.5 |
37.2 |
1.54 |
0.39 |
0 |
0.64 |
0.66 |
0.6 |
0.4 |
0.84 |
29Ts |
abundant diasteranes |
|||
Azal 1 |
YN0005 |
15.6 |
45.85 |
6900 |
Tithonian |
-28.2 |
-26.3 |
1.58 |
868 |
35.1 |
26.5 |
38.4 |
1.1 |
0.32 |
0.02 |
0.63 |
0.6 |
0.69 |
0.43 |
0.77 |
29Ts |
diasteranes |
|||
Marib Graben Seep |
YN0006 |
16 |
46 |
0 |
-26.9 |
-25.5 |
0.34 |
3929 |
46.6 |
28.6 |
24.8 |
0.13 |
0.13 |
0.01 |
4.45 |
0.14 |
1.05 |
0.61 |
0.34 |
low diasteranes -gammacerane |
|||||
Sarar 1X |
YN0007 |
14.9 |
50.4 |
L.Jurassic |
-26.8 |
-23.8 |
2.92 |
237 |
28.9 |
28.9 |
42.2 |
1.1 |
0.34 |
0.03 |
0.21 |
3.75 |
0.23 |
0.52 |
2.3 |
29Ts |
diasteranes |
||||
Sharmah 1X |
YN0008 |
14.8 |
50.176 |
7050 |
Eocene |
-23.3 |
-21.4 |
2.02 |
82 |
29.8 |
29.7 |
40.5 |
2.02 |
0.74 |
0.07? |
0.69 |
3.36 |
0.38 |
0.7 |
2.25 |
26Ts |
diasteranes |
|||
Shabwa 1 |
YN0009 |
15.4 |
47.03 |
6955-7162 |
U.Jurassic |
-28.6 |
-27.1 |
1.68 |
436 |
38 |
26.5 |
35.5 |
1.13 |
0.38 |
0.01 |
0.08 |
1.9 |
0.66 |
0.45 |
2.39 |
29Ts |
diasteranes |
|||
Shabwa 2 |
YN0010 |
15.4 |
47.03 |
7628' |
U.Jurassic |
-28.4 |
-26.8 |
1.62 |
288 |
35.3 |
26.7 |
38 |
2.46 |
0.54 |
0.02 |
0.05 |
3.08 |
0.65 |
0.36 |
3.03 |
29Ts |
diasteranes |
|||
Shabwa 2 |
YN0011 |
15.4 |
47.03 |
8202' |
M.Jurassic |
-28.6 |
-27 |
1.49 |
600 |
37.5 |
26.2 |
36.3 |
1.17 |
0.38 |
0.01 |
0.08 |
2.09 |
0.66 |
0.44 |
2.48 |
29Ts |
diasteranes |
|||
Sunah 1 |
YN0012 |
15.8 |
49.049 |
5575 |
L.Jurassic |
-29.3 |
-28.1 |
1.72 |
290 |
34.3 |
25.2 |
40.6 |
1.65 |
0.62 |
0.01 |
0.08 |
3.3 |
0.71 |
0.51 |
3.27 |
diasteranes |
||||
Hemiar 1 |
Yn0013 |
15.8 |
49.236 |
5425 |
L.Cretaceous |
-28.8 |
-27.8 |
1.69 |
545 |
35.2 |
24.1 |
40.8 |
1.12 |
0.42 |
0 |
0.07 |
2.98 |
0.71 |
0.55 |
2.82 |
diasteranes |
||||
Camaal 4 |
YN0014 |
15.7 |
49.053 |
5614-5650 |
Barremian |
-29.5 |
-28.3 |
0.91 |
385 |
34.7 |
24.6 |
40.7 |
1.56 |
0.57 |
0.01 |
0.08 |
3.7 |
0.73 |
0.46 |
3.59 |
diasteranes |
||||
Hahmade seep |
YN0016 |
16.2 |
48.16 |
surface |
-29.37 |
-28.21 |
1.26 |
8 |
6.3 |
83.2 |
10.5 |
5.71 |
1.01 |
0.5 |
5.23 |
0.36 |
0.7 |
0.1 |
??? |
||||||
Shabwa salt mine 1 |
YN0017 |
surface |
Upper jurassic |
-30.01 |
-29.3 |
-27.9 |
-28.7 |
0.26 |
533 |
13.7 |
28.2 |
58.1 |
0.32 |
0.12 |
0 |
0.52 |
0.12 |
0.83 |
0.47 |
0.03 |
no diasteranes |
||||
Shabwa salt mine 2 |
YN0018 |
surface |
Upper Jurassic |
-29.3 |
-27.7 |
-25.8 |
-28.1 |
1267 |
37.9 |
18.8 |
43.3 |
0.17 |
0.07 |
0 |
0.99 |
0.03 |
0.52 |
0.73 |
0.02 |
no diasteranes |
|||||
Mousabah 1 |
YN0019 |
15.3 |
45.9613 |
12090 |
-28.2 |
-26.2 |
1.68 |
69 |
36.1 |
25.9 |
37.9 |
2.16 |
0.51 |
0.01 |
0.65 |
1.28 |
0.45 |
0.36 |
1.5 |
||||||
Mousabah 1 |
YN0020 |
15.3 |
45.9613 |
12434 |
-28.2 |
-26.3 |
1.65 |
76 |
36.4 |
24.9 |
38.7 |
2.22 |
0.52 |
0.013 |
0.61 |
0.35 |
1.3 |
||||||||
Qâni' |
1265 |
surface |
-26.5 |
-85 |
1.02 |
14.3 |
31.2 |
54.5 |
0.26 |
0.16 |
0 |
0.44 |
0.23 |
0 |
no diasteranes |
||||||||||
Qâni' |
1269 |
surface |
-25.7 |
-81 |
0.24 |
27 |
31.3 |
41.7 |
0.73 |
0.22 |
0.07 |
0.15 |
0.56 |
0.58 |
diasteranes |
||||||||||
Qâni' |
1279 |
surface |
-27.1 |
-70 |
0.34 |
20.6 |
35.9 |
43.5 |
0.34 |
0.17 |
0.08 |
1.09 |
0.45 |
0 |
no diasteranes |
||||||||||
Qâni' |
1281 |
surface |
-27.5 |
-69 |
0.75 |
27.1 |
24 |
49 |
0.15 |
0.1 |
0.04 |
0.55 |
0.24 |
0 |
no diasteranes |
||||||||||
A'Rumah-Balhaf |
1393 |
surface |
-26.3 |
-86 |
1.15 |
1.61 |
2.1 |
0 |
6 |
2.13 |
no diasteranes-tricyclic terpanes-extremely degraded |
||||||||||||||
Harubya-Balhaf |
1394 |
surface |
-24.9 |
-76 |
1.1 |
1.5 |
8.1 |
0.77 |
no diasteranes-extremely degraded |
||||||||||||||||
Minqah |
1332 |
surface |
-26.4 |
0.55 |
no steranes and terpanes - |
Table 4 Molecular composition on crude oils, oil seeps and some bitumen from Qâni’
Figure 8 Comparison of alkanes from a frankincense (#1261) and a bitumen (#1279) sample from Qâni’. m/z 191 and 217 do show occurrence of terpanes and steranes in #1279 but terpenes in #1261. m/z 85 which represents n-alkanes, show high molecular weight n-alkanes with a slight odd-even predominance in the #1261 frankincense due to its vegetal origin. N-alkanes are restricted to C14-C18 in the bitumen and are associated with pristane and phtytane as currently seen in petroleum.
Figure 9 Location of oil seeps and crude oil that was used as references for the bitumen identified at Qâni’.
Figure 11 Gross composition of the dichloromethane extract in a ternary diagram: % sat.+ aro., % resins, % asphaltenes.
The GC-MS analysis of alkanes of the four samples from Qâni’, which were expected to be bitumen-bearing, confirms the occurrence of bitumen that exhibit markedly different origins. The #1269 ozokerite is separate from other Qâni’ samples with abundant diasteranes (cf. m/z 217, Figure 12) and the occurrence of 18α(H)-oleanane (cf. m/z 191, Figure 12). Other archaeological samples show various degrees of biodegradation in steranes and terpanes (Figure 12 & Figure 13) and changes in diagnostic ratios (Table 5) such as Ts/Tm, Gammacerane/C30abhopane, C29αααR/C29αααS, etc. suggesting different potential sources which are researched using data from oil seeps that were analyzed and by referring to data on crude oil from various fields (Figure 9). Selective biodegradation of the C29αααR, C28αααR and C27αααR steranes, which are the steranes with a biological configuration (#1281, Figure 13) were noticed among the recorded properties of biomarkers. This feature, which was also observed in other archaeological bitumen has been reproduced under laboratory conditions using Nocardia and Arthrobacter genera.28 A plot of several molecular ratios currently used for correlation purposes, i.e. 27Sdia/29αααR vs. Ts/Tm (Figure 14a), GA/31αβHR vs. Ts/Tm (Figure 14b), 18α(H)-oleanane vs. Ts/Tm (Figure 14c), and a ternary diagram with ββ-steranes (Figure 14d) did not show a match with either oil from oil fields or oil seeps especially Balhaf, which was, according to its location, thought to be the likely source. Diasteranes were lacking in three samples (Figure 15a) from Qâni’, but were abundant in all crude oil. The ozokerite (#1269) possesses special properties: abundant diasteranes, higher Ts/Tm (Figure 14a & Figure 14c) and the occurrence of 18α(H)-oleanane (Figure 14c). This last characteristic suggests that #1269 may have originated from Khuzistan in Iran.23
Figure 12 Mass fragmentograms of steranes (m/z 217) and terpanes (m/z 191) of samples #1265 and #1269: identification of bitumen.
Figure 13 Mass fragmantograms of steranes (m/z 217) and terpanes (m/z 191) of samples #1279 and #1281: identification of bitumen.
Figure 14 Mass fragmantograms of steranes (m/z 217) and terpanes (m/z 191) of samples #1279 and #1281: identification of bitumen.
Sample number |
location |
TOC (%) |
EO (%) |
sat. (%) |
aro. (%) |
pol. (%) |
sat. (%) |
aro. (%) |
res.(%) |
asp.(%) |
δ13Casp (‰ /VPDB) |
δDasp (‰ / SMOW) |
δ13Csat (‰ /VPDB) |
δ13Caro (‰ / VPDB) |
δ13Cres.(‰ / VPDB) |
δ13Cextract (‰ / VPDB) |
δDextract (‰ / SMOW) |
|
961 |
Qâni' |
67.6 |
95.2 |
0.8 |
17.3 |
81.9 |
0.7 |
21.6 |
75.3 |
2.4 |
-26.7 |
|||||||
962 |
Qâni' |
31.1 |
9.7 |
1.8 |
10.6 |
87.6 |
1.2 |
14.9 |
39.3 |
44.6 |
-24.8 |
-100 |
||||||
963 |
Qâni' |
22.2 |
29.9 |
0.7 |
6.4 |
92.8 |
0 |
10 |
39.8 |
50.2 |
-25.5 |
-111 |
||||||
964 |
Qâni' |
7.6 |
11.1 |
0.02 |
2.9 |
97.08 |
0.1 |
2.3 |
52.5 |
45.1 |
-26.9 |
-144 |
||||||
1261 |
Qâni' |
25.5 |
30.2 |
0.3 |
8.98 |
90.72 |
1.6 |
9.2 |
30.4 |
58.8 |
-25.6 |
|||||||
1265 |
Qâni' |
6.5 |
14.7 |
4.4 |
4.8 |
90.8 |
1.7 |
2.2 |
15.2 |
80.9 |
-26.5 |
-85 |
||||||
1269 |
Qâni' |
72.8 |
46.5 |
30.8 |
27.1 |
42 |
25.2 |
24.9 |
30.2 |
19.7 |
-25.7 |
-81 |
||||||
1271 |
Qâni' |
49.1 |
99.9 |
0.01 |
16.05 |
83.94 |
0.3 |
6 |
54.5 |
39.2 |
-25.9 |
-104 |
||||||
1279 |
Qâni' |
18 |
28.6 |
2.5 |
4.3 |
93.2 |
2.9 |
4.3 |
18.8 |
74 |
-27.1 |
-70 |
||||||
1280 |
Qâni' |
0.09 |
||||||||||||||||
1281 |
Qâni' |
17.8 |
23.4 |
1.8 |
5.6 |
92.5 |
0.6 |
2.5 |
14.8 |
82.1 |
-27.5 |
-69 |
||||||
1286 |
Qâni' |
73.7 |
0.01 |
4.49 |
95.5 |
0.4 |
5.4 |
49.4 |
44.8 |
-25.2 |
-104 |
|||||||
1393 |
Balhaf 1985-A'Rumah |
basement ? Infra-cambrian? |
13.3 |
22.3 |
64.4 |
13.3 |
22.3 |
35.7 |
28.7 |
-26.3 |
-86 |
|||||||
1393 |
Balhaf 1985-A'Rumah |
basement ? Infra-cambrian? |
16 |
28.3 |
44.3 |
16 |
28.3 |
28.4 |
27.3 |
-28.2 |
-27.2 |
-112 |
||||||
1394 |
Balhaf 1985-Wadi Arubya |
Mukalla sanstone-Lower Cretaceous |
9.3 |
9.2 |
81.6 |
9.3 |
9.2 |
14.4 |
67.1 |
-24.9 |
-76 |
|||||||
1394 |
Balhaf 1985-Wadi Arubya |
Mukalla sanstone-Lower Cretaceous |
9.4 |
7 |
16.4 |
9.4 |
7 |
12.7 |
70.9 |
-27.7 |
-25.6 |
-93 |
||||||
1332 |
near Mintaq 1 |
oil-stained sand-Tertiary |
6.9 |
11.2 |
23.3 |
58.7 |
11.2 |
23.3 |
46.9 |
18.6 |
-26.4 |
-90 |
||||||
1822 |
Shabwa salt mine 1 |
Upper Jurassic |
18.7 |
20 |
13.1 |
33.1 |
20 |
13.1 |
13.8 |
53.1 |
-27.9 |
-30 |
-29 |
-28.7 |
||||
1823 |
Shabwa salt mine 2 |
Upper Jurassic |
14 |
11.2 |
1.7 |
12.9 |
11.2 |
1.7 |
8.9 |
78.2 |
-25.8 |
-29.3 |
-27.7 |
-28.1 |
Table 5 Gross composition and isotopic data on samples from Qâni’ and oil seeps
In summary, archaeological bitumen from Qâni’ did not obviously match the analyzed oil seeps of Yemen and may have been imported from Khuzistan and Fars in Iran as suggested by the occurrence of 18a(H)-oleanane. Such a trade link has been highlighted at the ruins of Al-Baleed near Salalah on the southern coast of Oman, near Yemen, through the analysis of caulking residues on some wooden planks, which date from the 11th-12th century, that were used in the walls of the Husn fortress. These planks are re-used materials that came from a sewn boat likely to have been Iranian in origin as shown by the abundance of 18a(H)-oleanane in the bitumen that was analyzed.
The plot of isotopic data of asphaltenes, dD vs. d13C in Figure 15, allowed three groups of samples to be distinguished:
Figure 15 (a) Plot of 27Sdia/29αααR vs. Ts/Tm: comparison of the bitumen of Qâni’ to oil seeps and oil from oil fields.
(b) Plot of GA/C31αβHR vs. Ts/Tm: comparison of the bitumen of Qâni’ to oil seeps and oils from oil fields.
(c) Plot of 18α(H)-oleanane vs. Ts/Tm: comparison of the bitumen of Qâni’ to oil seeps and oils from oil fields.
(d) Ternary diagram with ββ-steranes: comparison of the bitumen of Qâni’ to oil seeps and crude oils from oil fields.
The characterization of frankincense by GC-MS analysis of selected samples
Archaeological frankincense has only been identified in various samples originating from sites located in Egypt, Yemen, France and Belgium.29−33,30-32 This study offers a novel opportunity to add chemical information on preserved archaeological frankincense as well as remains from incense burners that were affected by thermal treatments of various intensities. Thirteen archaeological samples were selected for the research and identification of frankincense. They were analyzed by GC-MS (Gas Chromatography-Mass Spectrometry). Their chromatograms showed the presence of predominant triterpenic biomarkers with oleanane, ursane and lupane skeletons. The occurrence of boswellic and lupeolic acids and their O-acetyl derivatives indicate that these samples contain a triterpenic resin belonging to the Burseraceae family named olibanum (Boswellia spp.) or frankincense.34−42 Previous research demonstrated that it is possible to chemically distinguish Boswellia frereana, an endemic species from North Somalia, from other botanical species of Boswellia.43 Indeed, B. frereana has a dominant triterpenoid named 3-epi-lupeol whereas the other Boswellia species also contain this component but in lower concentration and in association with a larger triterpenic population. Moreover, the usual biomarkers (a- and b-boswellic and lupeolic acids and their O-acetyled derivatives) are not detected in B. frereana (Figure 16). Since 3-epi-lupeol was not the predominant component in the analyzed samples (Figure 17), B. frereana can be excluded as the source of the frankincense present in the sample set.
Chromatographic profiles of triterpenic structures differed in the various samples: the distinction mainly concerned the most polar compounds eluting between 45 and 50 min. The sample location and history of Qâni’ suggest that resinous materials may have been exposed to various degrees of heating resulting in thermal degradation such as combustion and burning processes that may have affected their original composition. Polar compounds were degraded into non-polar molecules via thermal alteration and oxidation. The altered samples may be distinguished according to their degree of degradation corresponding to the extent of the effects of combustion. The specific chemical markers of frankincense are a-(18) and b-boswellic (19) and lupeolic (20) acids and their corresponding O-acetyl-derivatives (21-23). Molecular structures are given in Appendix 1. These molecules can undergo chemical transformation and form degradation products, i.e. 24-noroleanane-3,12-diene (3), 24-norursa-3,12-diene (5) and 24-norlupa-3,20(29)-diene (6). They were identified by their mass spectra and by referring to specialized literature (Table 6, Appendix 1).43,44 Other degradation compounds were also characterized: these are not functionalized and have one or two double bonds. They were identified with the NIST library of mass spectra as olean-11-12(18)-diene (1), ursa-11,13(18)-diene (2), olean-12-ene (4) and urs-12-ene (7). These molecules were generated by dehydrogenation of triterpenic precursors. Aromatic compounds can also be formed by further dehydration.45

|
No |
Compound |
tR (min) |
Skeleton type |
R1 |
R2 |
Double bond(s) |
|||
|
1 |
oleana-11,13(18)-diene |
37.72 |
O |
H |
CH3 |
C11-C12 and C13-C18 |
|||
|
2 |
ursa-11,13(18)-diene |
37.92 |
U |
H |
CH3 |
C11-C12 and C13-C18 |
|||
|
3 |
24-noroleana-3,12-diene |
38.12 |
O |
H |
- |
C3-C4 and C12-C13 |
|||
|
4 |
olean-12-ene |
38.31 |
O |
H |
CH3 |
C12-C13 |
|||
|
5 |
24-norursa-3,12-diene |
39.12 |
U |
H |
- |
C3-C4 |
|||
|
6 |
24-norlupan-3,20(29)-diene |
39.32 |
L |
H |
- |
C3-C4 and C20-C29 |
|||
|
7 |
urs-12-ene |
39.57 |
U |
H |
CH3 |
C12-C13 |
|||
|
8 |
3-epi-β-amyrine |
41.49 |
O |
a -OH, b -H |
CH3 |
C12-C13 |
|||
|
9 |
3-epi-α-amyrine |
41.94 |
U |
a -OH, b-H |
CH3 |
C12-C13 |
|||
|
10 |
3-epi-lupeol |
42.07 |
L |
a -OTMS, b-H |
CH3 |
C20-C29 |
|||
|
11 |
ursa-9(11),12-dien-3-one |
42.91 |
U |
O |
CH3 |
C9-C11 and C12-C13 |
|||
|
12 |
β-amyrone |
43.54 |
O |
O |
CH3 |
C12-C13 |
|||
|
13 |
β-amyrin |
44.06 |
O |
a -H, b-OH |
CH3 |
C12-C13 |
|||
|
14 |
α-amyrone |
44.4 |
U |
O |
CH3 |
C12-C13 |
|||
|
15 |
α-amyrin |
44.61 |
U |
a -H, b-OH |
CH3 |
C12-C13 |
|||
|
16 |
lupenone |
44.66 |
L |
O |
CH3 |
C20-C29 |
|||
|
17 |
lupeol |
44.95 |
L |
a -H, b-OH |
CH3 |
C20-C29 |
|||
|
18 |
α-boswellic acid |
45.15 |
O |
a -OH, b-H |
CO2H |
C12-C13 |
|||
|
19 |
β-boswellic acid |
45.63 |
U |
a -OH, b-H |
CO2H |
C12-C13 |
|||
|
20 |
lupeolic acid |
45.75 |
L |
a -OH, b-H |
CO2H |
C20-C29 |
|||
|
21 |
O-acetyl α-boswellic acid |
48.55 |
O |
a-OAc, b-H |
CO2H |
C12-C13 |
|||
|
22 |
O-acetyl β-boswellic acid |
49.17 |
U |
a-OAc, b-H |
CO2H |
C12-C13 |
|||
|
23 |
O-acetyl lupeolic acid |
49.28 |
L |
a-OAc, b-H |
CO2H |
C20-C29 |
|||
Table 6 Triterpenoic compounds in archeological samples
By referring to the chemical composition of terpenic derivatives (Table 7), it may be possible to establish a classification of archaeological samples as a function of their degree of alteration. Samples #1270, #1258, #1259 and #1283 did not show any degradation compounds (1-7) and contained native biomarkers of olibanum (18-23), so could be considered as well-preserved frankincense. However, samples #962, #963, #1263, #1277 and #1292, were devoid of the O-acetylated frankincense biomarkers (21-23) but their degradation derivatives (1-7) were ascribed as burned olibanum samples. In addition, a third class was defined with resins that contained a- (18), b-boswellic (19) and lupeolic (20) acids as well as O-acetylated derivatives (21-23) and their altered corresponding compounds (1-7). This last class (#964, #1264, #1270, #1286, #1287) is interpreted as a partly degraded resin due to a mild thermal degradation and/or ageing processes.
Archaeological samples |
||||||||||||||
No |
Compound |
962 |
963 |
964 |
1286 |
1270 |
1277 |
1292 |
1258 |
1259 |
1263 |
1264 |
1287 |
1283 |
1 |
oleana-11,13(18)-diene |
√ |
√ |
- |
- |
- |
√ |
√ |
- |
- |
√ |
√ |
√ |
- |
2 |
ursa-11,13(18)-diene |
√ |
√ |
- |
- |
tr |
√ |
√ |
- |
- |
√ |
√ |
√ |
- |
3 |
24-noroleana-3,12-diene |
√ |
√ |
√ |
- |
tr |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
- |
4 |
olean-12-ene |
√ |
√ |
- |
- |
√ |
√ |
√ |
- |
tr |
√ |
√ |
√ |
- |
5 |
24-norursa-3,12-diene |
√ |
√ |
√ |
- |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
- |
6 |
24-norlupan-3,20(29)-diene |
√ |
√ |
- |
- |
√ |
√ |
√ |
- |
- |
- |
- |
- |
- |
7 |
urs-12-ene |
√ |
√ |
√ |
- |
- |
- |
tr |
- |
tr |
√ |
√ |
tr |
- |
8 |
3-epi-β-amyrine |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
9 |
3-epi-α-amyrine |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
10 |
3-epi-lupeol |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
11 |
ursa-9(11),12-dien-3-one |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
12 |
β-amyrone |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
tr |
tr |
√ |
√ |
√ |
13 |
β-amyrin |
√ |
tr |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
- |
√ |
√ |
√ |
14 |
α-amyrone |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
15 |
α-amyrin |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
tr |
√ |
√ |
|
16 |
lupenone |
√ |
√ |
tr |
tr |
√ |
√ |
√ |
- |
- |
tr |
- |
- |
- |
17 |
lupeol |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
tr |
- |
√ |
√ |
√ |
18 |
α-boswellic acid |
- |
- |
√ |
√ |
√ |
√ |
tr |
√ |
√ |
- |
√ |
√ |
√ |
19 |
β-boswellic acid |
- |
- |
√ |
√ |
√ |
- |
- |
√ |
√ |
- |
√ |
√ |
√ |
20 |
lupeolic acid |
- |
- |
√ |
√ |
√ |
tr |
- |
√ |
√ |
- |
√ |
√ |
√ |
21 |
O-acetyl α-boswellic acid |
- |
- |
tr |
- |
√ |
- |
- |
√ |
√ |
- |
- |
tr |
√ |
22 |
O-acetyl β-boswellic acid |
- |
- |
tr |
- |
√ |
- |
- |
√ |
√ |
- |
- |
tr |
√ |
23 |
O-acetyl lupeolic acid |
- |
- |
tr |
- |
√ |
- |
- |
√ |
√ |
- |
- |
tr |
√ |
Table 7 Analytical data on terpenoid compounds
Apart from frankincense, fatty acids -myristic, palmitic and stearic acids- were identified in a number of other archaeological samples, i.e. #1258, #1263, #1264 and #1283 however their presence was not significant due to low concentrations. Their origin is questionable as they may originate from either from plants or animals that were incorporated with the minerals added to the frankincense. As discussed above these samples contained calcium carbonate and sulphate, and were anthropic mixtures that explain the occurrence of fatty acids as possible contaminants.
Analysis of three of the bitumen samples (#1265, #1279 and #1281) was undertaken using the frankincense flowchart in order to check if they were mixed with other ingredients, especially frankincense. The resulting chromatograms of samples #1265 and #1279 showed the occurrence of molecules with an hopane skeleton between 35-42 min in m/z 191 (Figure 18) but no other diagnostic terpenes. The bitumen samples that were analyzed were not therefore mixtures including frankincense but were mainly archaeological bitumen as found elsewhere.23 Sample #1281 is slightly different with the occurrence of the same hopane derivatives as well as triterpene close to epi-lanosterol (tR = 42.99 min), a-amyrin (15, tR = 44.86 min) and lup-12-en-3-ol (tR = 45.32 min), characterized with m/z = 189 and 203 and which may have originated from the introduction of vegetal matter into the bitumen (Figure 18). The presence of α-amyrin indicated the occurrence of a triterpenic resin belonging to the Burseraceae family.
Figure 19 GC-MS chromatograms (m/z 189+191+203) of the archaeological samples #1281, #1265 and #1279, between 35-45 min.
The results of the present study lead to the following observations
Firstly, the identification of bitumen in several samples is remarkable: a likely hypothesis is that it was used as a fuel and, when mixed with aromatic resin, facilitated fumigations related to religious practices. However, no such mixture was identified within the temple area. In addition, it is possible that the bitumen was used as a binder in elaborate mixtures to create diverse texture and density. Several samples were found to be mineral matter mixed with frankincense which was always the major constituent. It is important to underline that all samples that were analyzed were frankincense (Boswellia), never myrrh and it is significant that 35 samples of fumigation and offering residue found in religious complex were basically from the same resin and used in relation to a god or a religious practice. Thus, the use of some aromatics was likely to have been precisely defined. Fatty acids of either of animal or vegetal origin were identified in some mixtures indicating that anthropic mixtures comparable to those called now known as bukhur, associated to resins with other aromatic ingredients (flowers, wood, oils, etc.) were in use. Fumigations in a religious context could have been made with these preparations and not exclusively with pure resins.
Secondly, the analysis of bitumen samples shows that they did not originate from a local source (Balhaf or elsewhere) but probably from the Khuzistan area in Iran. The exchanges between Qâni’ and the Arabo-Persian Gulf are attested (mostly through the spread of pottery containers) but it is of particular interest to confirm the importance of a resource that was available locally. Bitumen was certainly available in the port of Qâni’ and reused from the waterproofing of boats. A dedicated trade of bitumen is attested by jars from south Mesopotamia which showed a thick deposit of Iranian bitumen when analyzed: these were excavated from coastal sites in eastern Arabia, including Qâni’.46
The geochemical study of thirty-one samples from Sector VII of Qâni’, which were excavated by the French Archaeological Mission and from the Sector VI by the Russian mission, has led to the identification of frankincense and four bitumen samples. The frankincense was fully preserved in the burnt-out warehouse excavated by Sedov and in the religious complex of Sector VII, and was partly altered by thermal treatments associated with incense burners. No other type of resins, such as copal from Africa as identified at Sharma, was found at Qâni’. Some samples were also analyzed for their mineral content and NaCl, gypsum and calcium carbonate were identified. In addition to frankincense, bitumen samples were found in potsherds in the religious complex. The use of the bitumen was not understood but may have been a substance for caulking ceramics or lighting fires in incense burners. The geological origin of the bitumen samples was not confirmed, despite a comparison with Yemeni crude oil and oil seep. More precisely, the bitumen samples did not match the Balaf oil seeps, which are not far from Qâni’. The occurrence of 18a(H)-oleanane in an ozokerite and in some other bitumens suggests an import from Iran, as was shown to be the case in Salalah with another set of samples.
The authors are indebted to the authorities of Yemen and especially to the General Organisation of Archaeology, Museum and Manuscripts who authorized the export of samples for analysis. John Zumberge who allowed us to use used the data on crude oil from Yemen, and Thomas Van de Velde who drew the maps. Thanks are also due to Jean-Bernard Berrut, Beatrice Ruiz, Marie-Hélène Doumergue and Daniel Dessort who carried out the analytical work leading to the characterization of bitumen. We are deeply indebted to Michael H. Engel for his comments and suggestions which were of significant value in the preparation of the manuscript. Helen Kirkbride and Tom Vosmer are highly acknowledged for their serious review of the manuscript and for their numerous suggestions and improvements to the text.
We are alsoss indebted to Michel Mouton who provided all the archaeological details related to the studied area and who collected the samples in the sanctuary of Qâni’.
Authors declare that there is no conflict of interest.

©2018 Connan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.