Journal of
eISSN: 2374-6947


Review Article Volume 3 Issue 1
Department of Medicine, Cardo Metabolic Institute, USA
Correspondence: Sunil J Wimalawansa, Department of Medicine, Cardo Metabolic Institute, USA
Received: January 15, 2016 | Published: February 23, 2016
Citation: Wimalawansa SJ. Vitamin D deficiency is a surrogate marker for visceral fat content, metabolic syndrome, type 2 diabetes and future metabolic complications. J Diabetes Metab Disord Control. 2016;3(1):6-13 DOI: 10.15406/jdmdc.2016.03.00059
Worldwide, epidemics of obesity, type 2 diabetes (T2D), metabolic syndrome, and vitamin D deficiency have emerged during the past three decades. These are in part due to changing of behavior and; poverty and the increased consumption of poor-quality, caloric-dense, cheap food; sedentary lifestyles; and less exposure to sunlight. Thus, these four diseases have some common origins. This article highlights the importance of serum vitamin D levels, the common link, in diagnosis and management of these four common disorders. Making a right diagnosis early would allow targeted, cost-effective interventions to reverse abnormalities and prevent the development of major complications that require expensive interventions later. Two such distinctive markers that need to be identified are visceral obesity and vitamin D deficiency. Such identification can be achieved simply by measuring standardized abdominal girth using a tape measure and serum 25 hydroxy vitamin D levels. These two surrogate markers are inversely correlated but not mutually exclusive. Thus, they can be used as clinically useful practical guidance, not only for screening but also for follow-up progress and effective management of the aforementioned disorders. The combination of having a higher waist circumference and lower serum 25 hydroxy vitamin D levels is an effective clinical tool for identifying those who are at a higher risk for the development of future metabolic complications.
Keywords: 25-hydroxyvitamin, Cardiovascular disease, Lipids, Premature mortality, Vitamin D insufficiency, Obesity
CDC, centers for disease control and prevention; BMI, body mass index; CVD, cardiovascular disease; NAFLD, non-alcoholic fatty liver disease; HDL, high density lipoproteins; LDL, low density lipoproteins
Worldwide, more than 1billion people have vitamin D insufficiency1,2 and an additional 750million have vitamin D deficiency.3,4 However, the reported prevalence of vitamin D deficiency varies from 10% to 70% in different countries.4?6 Because vitamin D deficiency is associated with multiple disorders, it has gained attention as a major public health problem.4 However, it is key nutritional deficiency that is easily and cost-effectively treated.6?8
The epidemics of obesity and type 2 diabetes (T2D) manifest in recent years result in part from excessive consumption of calories (mostly through calorie-dense fast food and fructose/corn syrup-based products and sugary drinks) in the presence of inadequate physical activities, the inappropriate and overuse of broad-spectrum antibiotics, chronic inflammation, infections, and diseases associated with poverty/low living standards.9?16
According to the Centers for Disease Control and Prevention (CDC), more than one-third of adults (34.9%) and 17% of youth in the United States are obese.17,18 The estimated annual medical cost related to obesity in the Unites States in 2012 was $190.2billion; which is nearly 21% of the nation’s annual medical spending. The estimated annual medical costs for obese people were one-third higher than those who were of normal weight,19,20 but the associated stigma discourages people from seeking treatment.21
Although obesity affects more than 600million people in the world, another 350million have undiagnosed obesity, particularly those with relatively normal body mass index (BMI) but who have intra-abdominal (i.e., visceral) obesity. Obesity is a global, preventable health problem of epidemic proportions and a major risk factor for chronic diseases, resulting in accelerated morbidity and premature mortality.
Obesity contributes to a host of non-communicable diseases, including cardiovascular disease (CVD), strokes, sleep apnea, depression, psycho-social problems, non-alcoholic fatty liver disease (NAFLD), inflammatory bowel disease (IBD), chronic kidney disease, increased risk for cancer, and osteoarthritis, and often leads to losses in productivity.12,13,16,22?25
In the United States, about one-third of adults are obese and another third overweight.20,26?29 Many persons with overweight or obesity have dyslipidemia, associated with raised serum levels of small density-low density lipoproteins (LDL), functionally impaired high density lipoproteins (HDL), and increased remnant particles secondary to reduced clearance of triglyceride-rich lipoproteins (raised triglycerides), which significantly increase CVD risks and events in persons with obesity.16,30 Despite the availability of various pharmaceutical agents, lifestyle modification is the principal approach to preventing weight gain and reducing obesity-related CVD and other risks.27,28,31,32
Measurement of visceral fat
Estimating visceral fat is important not only to identify individuals who are at higher risk for future CVD complications33,34 but also as a tool to identify those who are deficient in vitamin D. Anthropometric indices such as abdominal girth [waist circumference (WC)] and BMI have been used to categorize overweightness and obesity and explore relationships between obesity and vitamin D.2,34?41 However, the inability to distinguish visceral fat using the BMI precludes its usefulness for assessing abdominal fat contents or determining a therapeutic course in many ethnic groups.
The accuracy of identifying the visceral fat contents can be improved with newer techniques, such as bioelectrical impedance analysis, dual-energy x-ray absorptiometry, computed tomography, and magnetic resonance imaging.42?46 Nevertheless, these technologies are expensive, have associated radiation hazards, and are not cost-effective tools for screening the general population to identify and quantify visceral fat content in a clinically meaningful manner.47,48 Having higher WC and lower serum 25(OH)D levels is a better combined tool for identifying those who are at higher risk for future metabolic complications than is WC alone, WC plus lipid profiles, or expensive imaging techniques.
Obesity-importance of reducing visceral fat content
Obesity is a disease with major public health, social, and economic consequences that require serious attention from all stakeholders. Obesity is much more complicated than being just a lifestyle issue. In addition to the caloric imbalances, those who are genetically prone to accumulate weight have abnormalities of the mitochondria, which play central roles in energy expenditure and energy balance, and have genetic predispositions.39
Like other diseases, obesity has (A) a cause (caloric imbalance, availability and abundance of food, and consumption of low-nutritious, high-caloric food); (B) pathology (adipocyte-mediated excessive production of inflammatory cytokines and hormones); (C) pathophysiology (an environmentally and psychologically inducible dysregulation of appetite, low activity levels, body fat distribution, psychological issues, and deranged body-weight–controlling mechanisms),49 and (D) is a disease that can be treated (with anti-obesity medication).31,38
Although there are common causes, each individual has a different set of risk factors that lead to the development of overweight and obesity. Moreover, if the excess weight issue is untreated, many of those with obesity end up with serious complications.50?52 An effective strategy necessitates preventing individuals from becoming overweight and requires identification of the cause(s) of obesity in individual persons. Clinicians should have the ability to provide individualized care and treatment plans for their patients to assist them in losing weight and reducing complications.
Intra-abdominal (visceral) fat is a key site for generating inflammatory cytokines that lead to various metabolic abnormalities in the presence of obesity. This leads to a vicious cycle of systemic inflammation.53,54 Therefore, reducing intra-abdominal fat (using waist size is a surrogate marker) is a prime target in reducing insulin resistance and preventing future complications.
Decreasing the visceral fat burden is associated with improvements in most of the treatable abnormalities of metabolic syndrome, including hypertension, dyslipidemia, chronic inflammation, and development of T2D. Such approaches would not only minimize future serious metabolic complications but also improve quality of life and engender cost savings in the long run.
Visceral obesity and T2D
The prevalence of visceral obesity, T2D, and vitamin D deficiency increases with age. Increasing abdominal girth (measured as waist circumference) is a reasonable surrogate marker for abdominal obesity and excess visceral fat. Visceral adiposity is associated with CVD, metabolic syndrome, and T2D, particularly in ethnic minorities and Asians.38,55?57 Successful treatment in reducing visceral adiposity leads to reduction of the risk of T2D.58,59
In addition to deranged handling of glucose and free fatty acids, diabetes has an underlying generalized, chronic inflammatory status.60?62 Patients with T2D and chronic kidney disease have impaired endothelial function and vitamin D, and its analogs may play a role in regulation of endothelial function and inflammation.63 Insulin resistance, obesity, and T2D are associated with a marked increase in atherosclerosis coronary heart disease and stroke.64
Those with insulin resistance, obesity, and/or T2D have chronically elevated inflammatory markers, such as tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6), further supporting the underlying inflammation and oxidative stress. A similar etiology is existing with the chronic, hyper-caloric-malnutrition (obesity). These are thought to interfere with the anti-inflammatory effect of insulin, which in turn might promote inflammation.65 Because vitamin D has anti-inflammatory effects,66?70 it is not surprising that it has effects on improving blood sugar control, islet cell functions, insulin release, and decreasing insulin resistance.4,64,65
Visceral fat and metabolic syndrome
Metabolic studies have demonstrated that among equally obese patients, subjects with excess visceral adipose tissue have the metabolic profile with the highest risks.71,72 Accumulation of visceral adiposity precedes the development of type 2 diabetes in most ethnic groups.31 This is highlighted in a clinical study in Japanese Americans, which demonstrated an effect independent of fasting insulin, insulin secretion, glycaemia, total and regional adiposity, and family history of diabetes as a cause and a predictor for genesis of T2D.73,74
The correlation of abdominal adiposity has been reported with various components of metabolic syndrome, including anthropometric parameters and insulin resistance.55?57,75 Visceral fat is an organ that releases large number of harmful chemicals (cytokines) that lead to chronic inflammation, which particularly affects the liver and vascular system.4,6 Chemicals generated by hypertrophic visceral fat cells and macrophages reach the liver directly via the portal venous system, thus exposing it to a high contents of inflammatory agents that impair its functions, including derangement of glucose and fatty acid metabolism.
Rectification of vitamin D deficiency decreases insulin resistance
Several observational studies have demonstrated a consistent association of low serum 25(OH)D levels with diabetes, pre-diabetes, obesity, and metabolic syndrome.76,77 A population-based study from Norway confirmed a strong inverse association between elevated BMI and low serum 25(OH)D levels.78 In addition, the serum level of 25(OH)D is inversely correlated with the average blood sugar concentrations and insulin resistance.79
In patients with T2D, vitamin D supplementation increases insulin sensitivity, releases insulin, and decreases diabetes-associated chronic inflammation.80 Vitamin D stimulates insulin production in the pancreas and may play a role in preventing type 2 diabetes through its ability to decrease inflammation, insulin resistance, insulin synthesis and secretion,81 and perhaps decreased inflammation.69,70 Moreover, vitamin D and calcium intake are inversely associated with the risk of T2D.82 Those who consume three or more servings of vitamin D-fortified dairy products a day are at a lower risk for diabetes.83
Vitamin D also plays a role in insulin signaling84 and modifying the risks of diabetes.85?87 The findings of vitamin D receptors in pancreatic b cells88,89 further supports the notion that vitamin D influences insulin synthesis and secretion. Deficiency of vitamin D predisposes individuals to type 1 and type 2 diabetes and perhaps inadequate responses to anti-diabetes therapies.89?93
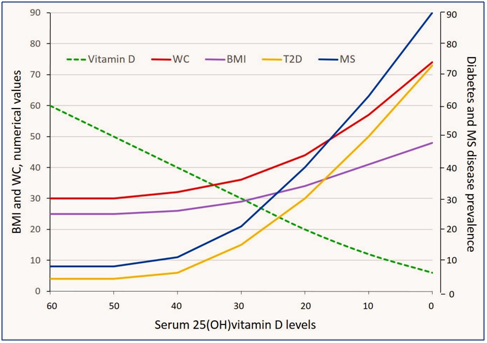
Figure 1 Inverse correlations of decreasing levels of serum 25(OH) vitamin D [25(OH)D] levels with the prevalence of type 2 diabetes (T2D) and metabolic syndrome (MS); the latter has the most predominant relationship. Negative correlations of 25(OH)D are higher with the waste circumference (WC; abdominal girth) in comparison to the body mass index (BMI). Direct or indirect associations of varying levels of serum 25(OH)D with four indices are presented in the figure. Graphic presentations are based on best estimates and construct on hypothetical basis (thus only in approximation). Broken green line represent the changing serum 25(OH)D levels.
Physiological functions of vitamin D
The traditional benefits of vitamin D are well recognized in the musculoskeletal system, including maintenance of calcium homoeostasis and bone mineralization.3,4,6,94,95 Vitamin D enhances intestinal calcium absorption and mineralization of osteoid tissues, thus increasing bone strength.7,96?98 Rickets in children and osteomalacia in adults are classic manifestations of severe vitamin D deficiency.4,97 Vitamin D also decreases the incidence of falls and thus fractures.4,97,99 Extra skeletal functional benefits of vitamin D in various non-communicable diseases and infection control also have been reported.2,6,9
Epidemiologic and cohort studies suggest that low 25 hydroxyvitamin D [25(OH)D] affects numerous and diverse physiologic functions, such as control of cell growth (e.g., cancer cells), protection against autoimmune disorders and bacterial and viral infections, and neuro-muscular coordination. Low vitamin D levels may worsen certain disorders, including cancer, metabolic syndrome, obesity, T2D, infectious diseases, and autoimmune disorders. Whether increased incidences of these diseases are consequences of widespread vitamin D deficiency is not clear. However, many of the reported relationships between vitamin D deficiency and diseases are based on epidemiologic observations.
Measurement of serum 25(OH)D is the best way to evaluate vitamin D status. Serum 25(OH)D levels of less than 20ng/mL are considered deficient, whereas optimum (physiological) blood levels are between 30 and 50ng/mL. To achieve such levels, an additional 1,000IU of vitamin D per day is sufficient for most lighter-skinned individuals, whereas elderly, obese, and dark-skinned individuals and other vulnerable groups of persons may need an additional 2,000IU/day or more to maintain physiologic serum 25(OH)D levels.
Vitamin D inadequacy either precipitates or exacerbates several chronic diseases, including autoimmune disorders, insulin resistance, diabetes, and cardiovascular disease.33,100 Other conditions also are linked to excess visceral fat.75 Multiple mechanisms have been proposed for the observed relationship between obesity and vitamin D.102,103
Altered vitamin D metabolism, abruption irregularities, and dilution of vitamin D within the excess fat mass of persons with obesity are some of the explanations provided for the low vitamin D status and need for larger doses of vitamin D supplements to increase serum vitamin D levels. Thus, vitamin D replacement therapy needs to be adjusted for body size to achieve the desired serum 25(OH)D concentrations.103 Figure 2 illustrates correlations between vitamin D and obesity, T2D, metabolic syndrome, and osteoporosis.
Many studies have reported an inverse association between serum 25(OH)D levels and visceral adiposity102,104,105 (Figure 1) (Figure 2). The percentage of total body fat is also inversely correlated with the serum 25(OH)D levels.102,104,105 Obese individuals have decreased bio-availability of vitamin D, thus necessitating higher doses of vitamin D.102 Obese patients require two to four times greater vitamin D supplements to normalize their serum 25(OH)D levels than do non-obese patients. African-American patients with vitamin D deficiency have an additional inverse association with the amount of visceral fat and calcified atherosclerotic plaques.106,107
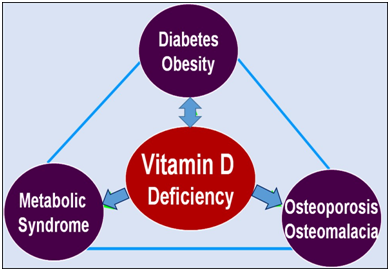
Vitamin D deficiency, visceral fat and obesity
Vitamin D inadequacy is a risk factor for obesity (and vice versa) and related to associated disorders, including insulin resistance, hyperlipidemia, hypertension, diabetes, and sleep apnea, leading to increased incidence of CVD.38,75,108?117 Those with the diagnosis of metabolic syndrome have significantly lower levels of serum 25(OH)D levels compared with those without the syndrome (Figure 1).100,115?117 Excess visceral adiposity is positively correlated with insulin resistance, T2D, certain cancers, and premature deaths.118 Evidence-based research on lifestyle interventions has demonstrated the effectiveness of such interventions in reducing insulin resistance,129 CVD, heart failure, stroke, cancer, diabetes, and all-cause mortality.120,121
Although the mechanism for the association between obesity and vitamin D insufficiency is not well understood4,97 researchers have suggested various mechanisms. Those who are obese are more likely to have vitamin D insufficiency,40,102 in part because of the combination of increased sequestration of vitamin D in excess visceral adipose tissue and dilutional4,6 effects, but other mechanisms are also likely.102,103,116,117 In addition, obese individuals need higher doses of vitamin D taken more frequently to attain and maintain their serum 25(OH)D levels than do individuals with normal BMI and normal waist circumference (WC).2,33,35,36,38
Association of serum 25(OH)D levels with visceral fat content
A number of studies have reported an inverse association of serum 25(OH)D levels and visceral body fat content: the higher the amount of visceral body fat, the lower the circulating 25(OH)D levels.123,124 Moreover, ethnic minorities and persons with darker skin have a higher prevalence of vitamin D insufficiency than do their lighter-skinned counterparts125 and are also known to have a high prevalence of abdominal obesity even with normal BMI. In addition to visceral fat, smoking, alcohol consumption, time spent outdoors, physical activity, occupation, menopausal status, intensity of skin color, cultural habits, exposure to solar ultraviolet B rays, and vitamin D supplement intake affect the serum 25(OH)D levels.4,97,126
There is a high prevalence of vitamin D deficiency in those who are obese2,46 and have metabolic syndrome.100 Serum 25(OH)D levels not only provide surrogate information on visceral obesity40 but also are a predictor of the levels of its active hormone, serum 1,25-dihydroxyvitamin D, in overweight and obese patients.33,34,36,127 Vitamin D metabolism, storage, and biological action are all influenced by the visceral adiposity content. Higher visceral fat content is associated with a higher incidence of vitamin D deficiency. Figure 3 demonstrates the relationships of vitamin D deficiency with insulin resistance, metabolic syndrome T2D and obesity.
Association of vitamin D and insulin resistance
There is a strong association of vitamin D deficiency with insulin resistance, particularly in those with high visceral fat.38,44 In addition, the amounts of visceral fat positively correlate with increase prevalence of impaired fasting glucose levels, abnormality of lipid metabolism, insulin resistance, hypertension, T2D, and a number of other metabolic risk factors.4,38,97,110?114,128,129
High visceral fat content is positively correlated with vitamin D insufficiency and deficiency. Thus, the serum 25(OH)D levels can be used as a surrogate marker to quantitate functional visceral fat contents and together with the WC (or any of the quantitative methods for estimating visceral fat content) for estimating future risks of metabolic complications associated with obesity (Figure 1) (Figure 3).55?57
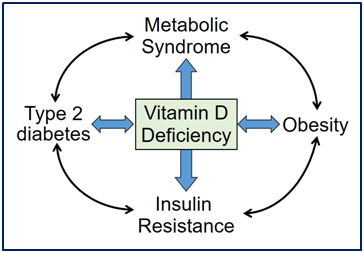
Thus, the combination of the measurements of WC and serum 25(OH)D levels can be used to increase the sensitivity of detection and screening of people suspected of having excess visceral fat. Serum 25(OH)D and WC are easily measureable surrogate markers for identification of those with excess visceral adiposity and perhaps are a cost-effective aid in the identification of future metabolic risk associated with abdominal obesity and T2D.
Association of vitamin D deficiency and metabolic syndrome
Metabolic syndrome is a constellation of metabolic abnormalities, including abdominal obesity, insulin resistance, hypertension, prothrombotic profile, and chronic inflammation, that collectively increase the risks of T2D and associated complications, including CVD and premature death.130?132 In most countries, the number of people with the aforementioned abnormalities has been increasing for the past three decades; such abnormalities are estimated to affect more than 40% of the US population.
Prolong vitamin D deficiency in the presence of excess visceral fat, increases calcium influx into adipocytes, further enhancing lipogenesis and secondarily, increase the production of parathyroid hormone.133?135 The latter is a physiological attempt to correct vitamin D deficiency.2,127 With reference to clinical studies that have used standard fixed doses of vitamin D supplementation in people with normal vitamin D levels without measuring their pre- and post-serum 25(OH)D levels, have not been able to demonstrate beneficial effects of vitamin D supplementation.1,136?138
Compliance with instructions and medications is the key to successful weight loss; without compliance, the overall effectiveness of therapies for weight loss and improving metabolic syndrome is limited. Despite medical advances and the availability of new pharmaceutical agents, the prevention of obesity by lifestyle changes, healthy eating, and increased physical activity are the most cost-effective treatment and the fundamental basis for managing obesity. Positive lifestyle changes are essential for the longer-term success in weight maintenance of all persons who are obese, including those who embark on pharmacotherapy or bariatric surgery. Adherence to effective lifestyle changes with other medical means enables maintaining body weight at a lower physiological set point and minimizing the long-term complications of T2D and obesity.
Obese and overweight persons should be provided with advice and guidance on healthy lifestyle changes and the causes of weight gain in a given person, a weight-reducing diet plan to which the patient can adhere, a reasonable physical activity regimen, and monitoring of the patient’s progress. Since the diet provides little vitamin D, it is not that relevant to the vitamin D deficiency. However, the lifestyle is important, including outdoor leisure activities and sensible exposure to sunlight. Medications and bariatric surgery are effective but not the first set of options. Even when such treatments are offered, they must be complementary to lifestyle and behavioral changes.139
In high-risk populations, timely and effective interventions would significantly reduce the human, social, and financial costs, as well as productivity lost from complications associated with metabolic syndrome and/or obesity. Testing for serum vitamin D levels should become a routine part of the managements and health maintenance of persons with metabolic syndrome, T2D, obesity, and osteoporosis.
Moreover, testing and treating for vitamin D deficiency would provide a real opportunity not only for cost-effective management of the deficiency in those who are at high risk but also for improving the health status of vulnerable populations and minimizing the need for universal supplementation. The use of serum 25(OH)D levels would increase the confidence of the diagnosis of visceral adiposity, allowing the taking of affirmative steps to rectify the problem. The combined use of abdominal circumference (WC) and serum 25(OH)D levels is more cost-effective in identifying or quantifying visceral adiposity and associated health risks than is relying on expensive lipid fraction studies and imaging methods.
None.
Author declares that there is no conflict of interest.

©2016 Wimalawansa. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.