Journal of
eISSN: 2374-6947


Short Communication Volume 10 Issue 1
1Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, India
2Scientific Director, Ex-Rotunda-A Centre for Human Reproduction
3Consultant Neurologist, Swami Satyanand Hospital, India
Correspondence: Dr Kulvinder Kochar Kaur, M.D, Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India
Received: February 13, 2023 | Published: May 26, 2023
Citation: Kaur KK, Allahbadia G, Singh M. Very low-calorie ketogenic diet (VLCKD)possesses further advantageous actions over’ Mediterranean diet in terms of better enhancement of gut microbiota (GM) in type2 diabetes mellitus patients- a short communication. J Diab Metab Disorder. 2023;10(1):48-54. DOI: 10.15406/jdmdc.2023.10.00252
There has been as escalating incidence of obesity &type2 diabetes mellitus (T2DM), &other co-morbidities for which diabesity had to be coined for this worldwide epidemic. All the newer combinations like Qsymia (topiramate, phentermine), Contrive (naltrexone: buprion), liraglutide etc have not been successful with cost prohibitions, or side effects/ contraindications. Thus attention had shifted to dietary therapies like Mediterranean diet (MD).Probiotic therapy. However none has been able for ensuring sustenance of weight reduction. We had reviewed in 2018 how the very low calorie ketogenic (VLCKD) diet might be successful not only in therapy of obesity but lot of correlated endocrine dysfunction. Subsequently we contrasted the effectiveness of VLCKD/ MD proving that VLCKD faired superiorly over MD. Furthermore, previously we had reviewed numerous publications on Gut Microbiota (GM) including role of Pro/ Prebiotics in obesity trials under way for NAFLD/ NASH/HCC/ type2 diabetes (T2D). Recently Deledda‘s group attempted a detailed assessment of alterations subsequent to VLCKD as well as MD. They contrasted various paradigms in 11 patients with recently diagnosed T2DM overweight/obesity patients following the VLCKD(KETO)or hypocaloric MD(MEDI) respectively; recording of paradigms at baseline(T0) &at two(T2) and three(T3) mths. The outcomes obtained -VLCKD possessed greater significant advantageous actions in contrast to MD over anthropometric paradigms, whereas biochemical enhancements were not statistically significant in2. Regarding GM , no significant outcomes were seen in alpha diversity ,beta diversity, and Firmicutes: Bacteroides ratio amongst the 2 groups in the KETO group, a significantly escalated advantageous taxa like the Verrumicrobiota phylum in addition to its members Verrumicrobiae and Verrumicrobiales, Akkermansiacea, Akkermansia, Christensellacea family, Eubacterium spp and decrease in microbial taxa prior correlated with obesity(Firmicutes along with Actinobacteriota)or other diseases(Allistipis) was seen at T2 as well as T3. Regarding MEDI group differences were restricted to a significant escalation in Actinobacteriota phylum at T2 as well as T3 as well as Firmicutes phylum at T3. Furthermore, a metagenomics change is correlated with certain metabolic pathway were seen only in the KETO group. Hence they concluded that both dietary strategies aided in enhancement of their health status, however VLCKD has illustrated greater advantageous outcomes regarding body makeup along with their GM paradigms. Their results showed VLCKD correlated with superior GM& good anthropometric changes -not seen with MD at 3 mth time point .Only limitation was 11 patients due to COVID arrival, hence no more recruitment, thus future studies warranted.
Keywords: mediterranean diet (MD), very low calorie ketogenic diet, gut microbiota, weight
Obesity by definition is body mass index (BMI) ≥ 30kg/m2,1 has assumed to be an escalating condition with a pathology that has not been fully clarified. World Health Organization (WHO) recently displayed that there is a trend of enhancement of this disease along with inability to fight it till now.2 Actually, 59% of European adults in addition to 1 in3 children (29%males as well as27% females) are overweight or have already generated obesity.2 Specifically by 2016, age standardized overweight/ obesity prevalence amongst adults in Italy was 58.5% along with 19% respectively.3 Intriguingly, along with obesity, inflammation runs in parallel, which gets modulated by the over generation of Reactive oxygen species (ROS).4–6 Additionally, obesity is correlated with multiple definition co-morbidities along with chronic diseases like, type2 diabetes mellitus( T2DM), hypertension, dyslipidemia, Polycystic ovary syndrome (PCOS), different cancers, sleep apnea syndrome of others.7,8 Despite weight reduction is no joke; basically aim is attaining decreased energy consumption along with escalating energy expenditure.9–11Different probable approaches exist regarding weight reduction inclusive of anti-obesity agents, bariatric surgery as well as variability of dietary manipulation.12–14 Any new antiobesity agents possessing advantageous properties are restricted in view of economically not viable besides certain contraindications along with probable inimical sequelae[15,rev in detailby us13 Though bariatric surgery usually possesses effectiveness with regards to weight reduction as well as aids in T2DM remitting besides metabolic syndrome (MetS); nevertheless, multiple complications, hence utilization of this procedure is feasible in selective subjects possessing robust obesity, without any contraindications for this surgery.16,17 Of the different nutritional strategies for obesity treatment is inclusive of high protein diet.18
Mediterranean diet (MD) portrays a healthy dietary style that includes different kinds of plant foods.19 Foods like extra virgin olive oil (EVOO), fruits, vegetables, legumes, nuts, red wines, along with whole grain cereals comprise the MD. Less saturated fat, greater mono- and polyunsaturated fats, bioactive compounds inclusive of polyphenols, as well as omega-3 fatty acids possessing antioxidant as well as anti-inflammatory qualities are its main properties. With the acknowledgement of its anti-inflammatory in addition to antioxidant characteristics. as well as some inherent traits (high ingestion of carbohydrates, fat, monounsaturated fatty acids, as well as fibre, are among other things) MD may be a good nutritional preference for those looking to lose weight.19 These advantageous MD actions are in view of numerous foods, anti-inflammatory along with antioxidant actions20 This MD is believed to be the best dietary model for reducing cardiovascular risk in view of it having advantageous biological actions correlated to anti-inflammatory, anti-hypertensive, anti-diabetic, along with anti-atherogenic actions that are attributed to the mode of action, in addition to trials correlated with intervention.21 Aside from the decrease in anatomical, allergy, along with asthmatic disorders, MD is further documented to be associated with a lesser incidence of some cancers.4,20,22–24 Additionally, MD has been correlated to the crosstalk of pollutants as well as the maintenance of environmental factors, both of which decline the incidence of cardiovascular disease (CVD) along with facilitate total health.25 The capacity of MD to positively modulate the gut microbiota (GM) makeup apart from diversity has further been confirmed,26 Specifically in patients with overweight or obesity.27 In case of T2DM, MD is advantageous in avoiding the progress of T2DM in view of its anti-inflammatory and antioxidant features and good alterations in GM.28
Ketogenic diet
Nevertheless, obesity issues continue in spite of the MD’s recognized impacts of advantage with regards to human health. Regarding this, people with obesity who did not lose weight using nutrition-correlated macronutrient balance therapies might get benefit from the VLCKD, a previously acknowledged approach.29,30 This diet’s components of carbohydrates are restricted (usually <30 g daily) along with, relatively enhanced protein (about 43% of full energy) fat around 44% of full energy). The total daily energy usage was pretty low (about 800 Kcal). By, a ketogenic diet (KD) is a diet that is defined as a diet which can cause ketosis. This refers to the existence of physiologically considerable blood ketone body quantities(equal to 4 mmol/L) in view of the diet’s influence on the liver’s over generation of ketone bodies (KB).31 As per the most recent example, it is efficacious in treating obesity, as well as dyslipidemia, and cardiovascular risk factors, as well as along with lower calorie ingestion, diminished insulin levels, escalated glucagon levels, and specifically the generation of ketone bodies, which have an influence in addition to other actions of advantage with more rapid weight loss compared to other dietary manipulation.30,32 It is possible to enter ketosis by fasting for an extended time or by dramatically decreasing daily carbohydrate ingestion (50 g)as per the project that got introduced by Blackburn. Utilizing VLCKD is to attain rapid weight decrease.36 The oxidation of fats stored as the body’s main energy source is made easier as a result of the enhanced caloric deficiency.37
Additionally, ketosis has the extra advantage of causing anorexia the effect of ketone bodies.38 Nevertheless, the effects of VLCKD on lean mass are not significantly different from those we get from other weight loss approaches39CVarious scientific societies have suggested the safety of VLCKD regarding the management of obesity, with or without T2DM, pointing out how this diet is correct in controlling metabolic paradigm in a similar or rarely even more beneficial in comparison to MD32,39 as well as have given guidelines with indications and contraindications. Although certain confusion among investigators is still existent.32 The effects of KD on GM have primarily been studied in animal models or cases of humans involving neurological and psychiatric illnesses, as well as infrequently in obesity and diabetes.40,41 Earlier Besides have reviewed numerous publications regarding Gut Microbiota(GM) inclusive of role of Probiotics and Prebiotics in obesity, therapies for NAFLD, trials under way for NAFLD/ NASH therapy; NAFLD/ NASH/HCC/type2 diabetes(T2D).43–46
The authors performed an exhaustive search on differentplatforms like PubMed; Google scholar; Web of Science; Embase; Cochrane review library utilizing the MeSH terms; m“Obesity/Overweight”; “Various diets MD”; “VLCKD; Gut Microbiota(GM) for assessment regarding the effectiveness of which diet is better in the context of GM” from 1995 till date.
We found a total of 1050 articles out of which we selected 73 articles for this review. No meta-analysis was done. Thus recently Deledda‘s group,47 decided to assess the Gut Microbiota (GM) in correlation of hypocaloric Mediterranean diet (MD) along with VLCKD. They contrasted anthropometric, biochemical, lifestyle paradigms, quality of life (QOL), GM in 12 patients with recent diagnosis of T2DM made in addition to overweight along with obesity being randomly allocated to the 2 groups comprising of 6 along with 6(1 eliminated in view of developing Covid ) patients following the VLCKD(KETO)or hypocaloric MD(MEDI) respectively; recording of paradigms at baseline(T0) along with subsequent to two(T2) as well as three (T3) months was done. The outcomes obtained illustrated that VLCKD possessed greater significant advantageous actions in contrast to MD over anthropometric paradigms, whereas biochemical enhancements were not statistically significant.
In detail subsequent to two months of dietary protocol, twenty-one bacterial taxa significantly escalated, whereas thirteen were significantly decreased; following three months of NI, twenty-two microbial taxa were significantly escalated, and ten were significantly eliminated. Furthermore, contrasting amongst GM communities at T2 as well as T3 time points illustrated that three taxa were enriched in T3 and five taxa were abundant in T2 (Figure 1). Results were ranked by their MaAsLin2 coefficient: the Verrucomicrobiota phylum was isolated in the form of major biomarker in KETO, in addition to its members Verrucomicrobiae, Verrucomicrobiales, Akkermansiaceae, as well as Akkermansia,both at T2 along with T3 of nutritional intervention; while within the Firmicutes phylum correlations were linked to maximum robustly Christensenellales order and Christensenellaceae family in the simultaneous time points. Simultaneously, the Actinobacteroidota phylum was significantly eliminated both at T2 and T3; whereas, amongst the Firmicutes phylum, genera from the Lachnospiraceae family (Agathobacter, Anaerostipes, Fusicatenibacter, and Dorea),to Ruminococcaceae family (Subdoligranulum) were significantlyeliminated as a sequel of two months of NI in KETO in addition to the Barnesiella as well as Butyricimonas genera (from the Bacteroidotaphylum as well as Bacteroidales order), the Lachnoclostridium as well as X Ruminococcus torques group genera (from the Firmicutes phylum and Lachnospiraceae family) were significantly diminished subsequent to three months of NI in the same patients in contrast to baseline Moreover, the UCG 010 family in addition to its unclassified members at the genus along with species level illustrated a strong association in KETO as a consequence of the NI from T2 to T3;by contrast, the genus Lachnoclostridium and a Lachnoclostridium unclassified species (from the Firmicutes phylum), the Tannerellaceae family and its members Parabacteroides and Parabacteroides distasonis (Bacteroidota phylum) were significantly correlated at T2 in contrast to T3 (Figure 1).
In the context of the MEDI group, they found that no taxa differed significantly subsequent to two months of the dietary protocol. The Actinobacteroidota phylum was isolated in the form of single taxon that increased after three months of NI compared to baseline; while, by comparing the GM communities of T2 and T3 time points, in addition to Actinobacteroidota, also robust correlations were associated with Firmicutes phylum at T3. The Desulfobacterota phylum along with a species from the genus Bacteroides were significantly correlated with T2 time points compared to T3 in the same patients (Figure1) Enrichment alteration amongst, time points can be further speculated by accounting the germane relative enriched variation along the nutritional intervening (Figure 2).
In view of max MaAsLin2 correlations for KETO occur at the genus level and most MaAsLin2associations for MEDI take place occur at the phylum level, (Figure 3) indicates significantly abundant or eliminated taxa at the genus and phylum levels for KETO and MEDI, respectively.
Comparative anticipation assessment of the functional metagenome was conducted usingPICRUSt2. A total of seventy significant metabolic pathways were isolated in KETO overtime (Figure 3). Specifically the frequent common twenty two pathways were significantly escalated both at T2 in addition to T3 contrasted with baseline, whereas the same seventeen pathways were significantly diminished in the simultaneous time points. Of the rest thirty-one pathways, eleven and six were significantly escalated as well as decreased, respectively, two months of NI and, similarly, following three months, despite with small effect size; seven and six were significantly escalated as well as diminished, respectively, subsequentto three months of NI as well as, simultaneously, after two months, despite with small effect-size. Additionally, only one pathway (phenylalanine metabolism) significantly diminished at T3 contrasted with T2, in parallel with a decrease in T2 as well as T3 contrasted with baseline, despite a small affect size. In KETO, the maximum robust correlations were positively associated to steroid biosynthesis, carotenoid biosynthesis, and non-homologous end-joining pathways both at T2 and T3compared with baseline, while penicillin and cephalosporin biosynthesis, limonene, and pinene degradation and ethylbenzene degradation pathways were strongly and negatively associated with the same time points. Moreover, among other robustly associated pathways, xylene degradation was significantly and negatively correlated at T2, whereas while at T3, it was decreased, although with a small effect size; carbohydrate digestion as well as absorption were the most s robustly and negatively correlated pathway with T3 contrasted with baseline, whereas while at T2 it was diminished, despite with small effect-size. No pathway was significantly correlated with MEDI over time. Regarding GM, absence of significant outcomes In the context of alpha diversity along with beta diversity, and Firmicutes: Bacteroides ratio amongst the 2 groups in the KETO group, a significantly escalated advantageous taxa like the Verrumicrobiota phylum in addition to its members Verrumicrobiae as well as Verrumicrobiales, Akkermansiacea as well as Akkermansia, Christensellacea family, Eubacterium spp along with decrease in microbial taxa prior correlated with obesity(Firmicutes along with Actinobacteriota)or other diseases(Allistipis) was seen at T2 as well as T3. Regarding MEDI group differences were restricted to a significant escalation in Actinobacteriota phylum at T2 as well as T3 as well as Firmicutes phylum at T3. Furthermore, a metagenomics change is correlated with certain metabolic pathway were seen only in the KETO group.Hence they concluded that both dietary strategies aided in enhancement of their health status, however VLCKD has illustrated greater advantageous outcomes regarding body makeup along with their GM paradigms (Figure 1–3).47,44
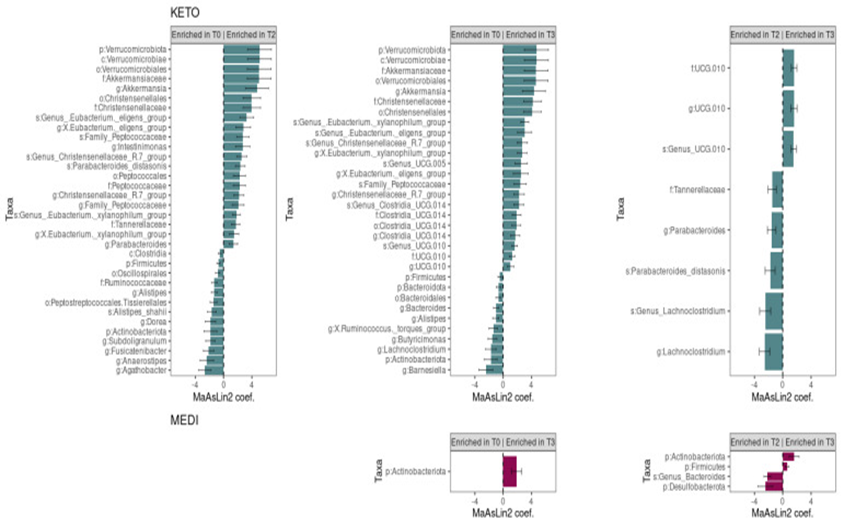
Figure 1 Courtesy ref no-47-Changes in gut microbiota taxa abundances between time points for each diet. Each subplot concerns a comparison between timepoints (T0 vs. T2, T0 vs. T3, or T2 vs. T3) in one of the diets (KETO, green MEDI, purple). Statistical significance was evaluated by running a Generalized Linear Mixed-effects Model with MaAsLin2. Effect size is represented by the MaAsLin2 model coefficients and respective standard errors. Only taxa abundance changes at p ≤ 0.05 and q ≤ 0.25 are considered statistically significant. q: p adjusted for Benjamini–Hochberg (BH) correction test with a cut-off at q ≤ 0.25. KETO = 6 patients who followed a very-low-calorie ketogenic diet (VLCKD), MEDI = 5 patients who followed a low-calorie Mediterranean diet (MD). Samples were analyzed at baseline (T0), after two months (T2), and after three months (T3) of nutritional intervention.
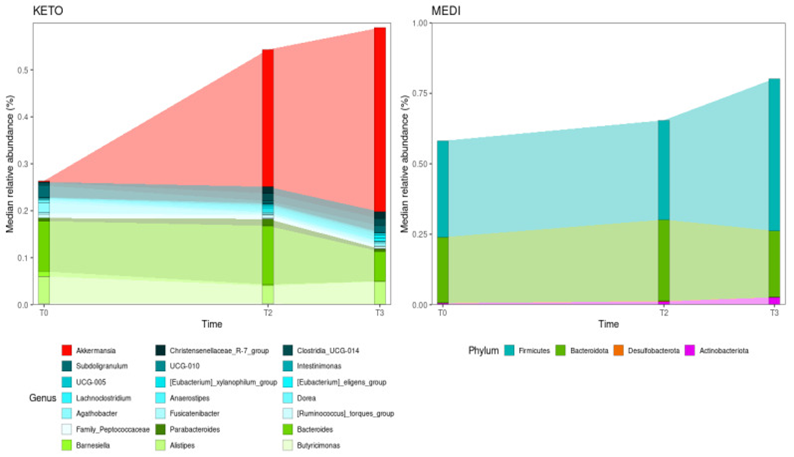
Figure 2 Courtesy ref no-47-Relative abundance changes in gut microbiota taxa between time points for each diet. Only taxa significantly enriched or depleted during the nutritional intervention (according to MaAsLin2 models) are shown (at the genus level for KETO and at the phylum level for MEDI). Genera are colored in the KETO plot based on the phylum to which each genus belongs (red: Verrucomicrobia; blue: Firmicutes; green: Bacteroidota). KETO = 6 patients who followed a very-low-calorie ketogenic diet (VLCKD), MEDI = 5 patients who followed a low-calorie Mediterranean diet (MD). Samples were analyzed at baseline (T0), after two months (T2), and after three months (T3) of nutritional intervention.
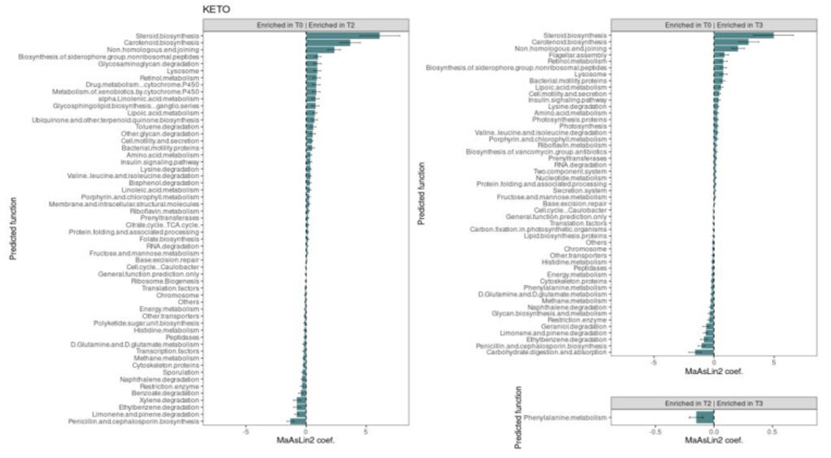
Figure 3 Courtesy ref no-47-Changes in gut microbiota predicted function abundances between time points for each diet. Each subplot concerns a comparison between time points (T0 vs. T2, T0 vs. T3, or T2 vs. T3). Statistical significance was evaluated by running a Generalized Linear Mixed-effects Model with MaAsLin2. Effect size is represented by the MaAsLin2 model coefficients and respective standard errors. Only predicted function abundance changes at p ≤ 0.05 and q ≤ 0.25 are considered statistically significant. KETO = 6 patients who followed a very-low-calorie ketogenic diet (VLCKD). Samples were analyzed at baseline (T0), after two months (T2), and after three months (T3) of nutritional intervention.
By attempting Comprehensive GM characterization through taxonomic analysis, by means of the Generalized Linear Mixed-effects Model, confirmed after multiple testing corrections, with a cut-off at q ≤ 0.25, they found various significant microbial markers associated with the NI, but almost exclusively with the ketogenic one. Results were ranked by their MaAsLin2 coefficient: the Verrucomicrobiota phylum was identified as the main biomarker in KETO, together with its members Verrucomicrobiae, Verrucomicrobiales, Akkermansiaceae, and Akkermansia, both at T2 along with T3 of NI. Intriguingly, these advantageous taxa illustrated a significant escalation of up to three months of nutritional intervention (NI) in KETO but not in MEDI, although in the former, the NI at the end of phase T3 analogous to the low-calorie MD of the MEDI group, while the T2 of KETO correlated with the end of the ketosis phases. Akkermansia muciniphila portrays the maximum assessed microorganism from these taxa, which is believed to be a significant biomarker of intestinal homeostasis, whose physiological action in facilitating intestinal intactness for its ability to stimulate the mucous turnover rate are well reported.48,49 A muciniphila aids in the intestinal health as well as glucose homeostasis48,50 as well as has been illustrated to enhance improve the metabolic status clinical outcomes after a dietary intervention in overweight/obese adults,51 along with have protective actions on diet-induced obesity.52,53 Furthermore, it has been posited to control adipose tissue metabolism as well as the accumulation of fat55 along with its escalation has been correlated with KD.54 A muciniphila supplementation in patients with overweight/obesity was correlated with diminished inflammation marker quantities as well as and enhanced various metabolic paradigms,56 whereas in animal models of diabetes and obesity restored the integrity of the epithelial mucosa, enhanced glucose tolerance, as well as metabolic paradigms.57 Conversely, its elimination has been correlated with many diseases, like inflammatory bowel disease along with metabolic disorders.58
As validation of their advantageous action, the Verrucomicrobia phylum, together with its members Verrucomicrobiaceae, Akkermansia, and Akkermansia muciniphila, was isolated as the major biomarker incentenarian subjects.59–61 Amongst the Firmicutes phylum, the correlations were also associated with the Christensenellales order and Christensenellaceae family both at T2 and T3 of VLCKD, as previously described.54 An unclassified genus and species from Christensenellaceae_R.7_group were also robustly correlated, although with a lowerMaAsLin2 coefficient. This comprises an interestingly, observation finding on the influence of the ketogenic diet on obese patients with T2DM, considering that a decrease in Christensenellaceae was seen in individuals with pre-type 2 diabetes.62 Christensenellaceae are implicated in the fermentation of proteins fibres as well as have been correlated with a diet low in refined sugars and high in fruit as well as and vegetables, with ingestion of dairy products and an escalation of in animal products in the diet.63 These confirmation evidences are in parallel with a protein and non-starchy vegetable intake in the first sixty days of the ketogenic diet in KETO, subsequently the slow steady introduction of fruit, dairy products, legumes, along with cereals until T3.Furthermore, Goodrich et al. illustrated that the inoculation of the obese human microbiome in germ-free mice fed a high-fibre diet induced a decrease in adiposity only in mice receiving fecal transplant modified with the addition of Christensenella minuta, in contrast to those receiving unmodified stools or stools containing non-viable C. minuta.64 Intriguingly, the Christensenellaceae family has been correlated with a lean phenotype, negatively associated with visceral fat mass, trunk fat, android fat,65 waist circumference, along with and waist/hip ratio;63 in addition to it is after a reduction in bodyweight in obese postmenopausal women following NI.66 These data are consistent with a significantly greater reduction in body weight, BMI, WC, and FM in our KETO cohort compared to MEDI, in which no significant enhancement in these bacteria taxa was. The Christensenellaceae family has also been negatively associated with dyslipidemia67,68 and positively associated with healthy glucose metabolism.69 In agreement, fasting blood glucose illustrated a greater enhancement, although not significant, in the KETO group than in MEDI; moreover, as regards the value of HbA1c, a more significant reduction was displayed in the KETO group subsequent to three months of NI found. Akin to the Verrucomicrobia phylum as well as its members, Christensenellaceae has further been correlated with human longevity,59,61,70–72 due to them being a marker of human health.
Their study implied the potential advantageous action of a VLCKD protocol in drug-naïve patients with T2DM as well as overweight/obesity, at least in the short term. Specifically, these advantageous actions common apparently are greater in contrast to those found with a canonical MD with regards to diminished weight elimination as well as and the influence on GM, despite future investigations are warranted. Actually, the VLCKD has illustrated greater improvements in anthropometric estimates (weight, BMI, FM%, and WC) in addition to in quality of life in contrast to the MD. However, alteration in metabolic variables was not statistically significant among the two diets. The outcomes further highlighted an improvement in eating habits, with an escalated adherence to MD in both groups. The continued shift to a Mediterranean-style diet subsequent to two months of KD aids patients not to be excessively restrictive about many food groups as well as decrease their carbon footprint. Still, physical activity continued to be unaltered in both groups. In agreement their observation highlights a greater advantageous influence of the VLCKD on the intestinal microbial phenotype, repointing that this diet might be believed to be an appropriate therapeutic strategy in managing newly diagnosed diseases without drugs. Overall, in the KETO group, both after two months (ketosis phase) along with subsequent 3 months of NI (shift to MD), there was a significant escalated biomarkers of intestinal homeostasis, like Verrucomicrobiotaphylum with its members Verrucomicrobiae, Verrucomicrobiales, Akkermansiaceae, and Akkermansia, as well as in advantageous microbial taxa associated with a lean phenotype in addition to with ahealthy glucose metabolism, like the Christensenellaceae family and in taxa capable of modulating gut inflammation via SCFAs generation (Eubacterium spp.); whereas the decrease in microbial taxa priorly correlated with obesity (Firmicutes and Actinobacteriota) or other diseases (Alistipes) was observed. Greater clarity must be made regarding the correlation of the Peptococcaceae family in view of the discordant data in the literature on their beneficial role. The ketosis phase (T2) was associated with the reduction in taxa belonging to theLachnospiraceae family, in taxa correlated with GIT neoplasms and poor metabolic control (Subdoligranulum) and with the increase in taxa already shown to be enriched in diabetic. Limitations were only 12 enrolled patients in view of COVID pandemic onset so stopped enrolling.
None.
The authors declare there is no conflict of interest.

©2023 Kaur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.