Journal of
eISSN: 2374-6947


Research Article Volume 3 Issue 6
1Department of Physical Medicine and Rehabilitation, Kaohsiung Veterans General Hospital, Taiwan
10Department of sport, Health and eisure, Chung Hwa University of Medical Technology, Taiwan
2Department of Biological Science and Technology, Chung Hwa University of Medical Technology, Taiwan
3Graduate Institute of Biomedical Science, National Sun Yat-sen University, Kaohsiung, Taiwan
4Graduate Institute of Medical Laboratory Science and Biotechnology, Chung Hwa University of Medical Technology, Taiwan
5Department of Internal Medicine, Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan
6Graduate Institute of Biomedical Science, Chung Hwa University of Medical Technology, Taiwan
7Department of Food nutrition, Chung Hwa University of Medical Technology, Taiwan
8Department of Medical Technology, Kaohsiung Veterans General Hospital Tainan Branch, Taiwan
9Department of Public Health, National Taiwan University, Taiwan
Correspondence: Yang Yu-Lin, Graduate Institute of Medical Laboratory Science and Biotechnology, Chung Hwa University of Medical Technology, Taiwan, Tel +886 6 267 7250, Fax +886 6 267 7250
Received: July 07, 2016 | Published: October 28, 2016
Citation: Wang JL, Chen YJ, Hsieh PF, et al. Verbascoside reverses TGF-? 1-induced renal cellular fibrosis. J Diabetes Metab Disord Control. 2016;3(6):122-127. DOI: 10.15406/jdmdc.2016.03.00083
Background: Renal fibrosis is characterized by interstitial cells hypercellularity and matrix protein accumulation, cause of loss normal function and finally kidney failure. Transforming growth factor-β (TGF-β) is a profibrotic cytokine as a key mediator for Smads pathways. Verbascoside is a pure compound most from Osmanthus spp. In addition, Verbascoside has an antioxidants, anti-inflammatory and immunosuppressive functions. In here, we investigated the underlying mechanism of Verbascoside in the regulation of TGFβ1-induced cellular fibrosis in NRK-49F.
Materials and Methods: NRK-49F (Rat kidney fibroblast Cells) were cultured in TGF-β1 (5ng/ml) for days. The cells treatment with different concentrations of Verbascoside (0.1mM, 1mM, 10mM) in last 24 hours. The effect of Verbascoside on cell viability in renal fibrosis by MTT test. Here we evaluated the in vitro cytotoxic effect of Verbascoside in renal fibrosis using the LDH release assay. We determined to EMT relevant markers including fibronectin and TGF-β1/Smads transducer including Smad4, Smad2/3 and Smad7 by Western blot assay. The expression of the extracellular fibronectin by ELISA. Results: MTT analysis showed that Verbascoside did not significantly affect levels of cell viability. Treating cells with Verbascoside did not release a significant amount of LDH compared to control. Western blotting showed that Verbascoside dose-dependent reverse TGFβ1-induced expression of fibronectin in NRK49F cells. Immunofluorescence assay showed that Verbascoside (10mM)) reverse TGFβ1-induced expression of fibronectin in NRK49F cells.
Conclusion: We propose that Verbascoside is a potential fibrosis antagonist for renal cells by TGF-β1/Smads signaling pathway.
Keywords: renal fibrosis, tgf-β1, verbascoside, fibronectin, tgf-β1/smad signaling pathway
Renal interstitial fibrosis is the common feature of all forms of renal failure, is characterized by interstitial cells hypercellularity and matrix protein accumulation, considered the hallmark of progressive renal disease.1-3 The important evaluating of fibrogenesis is fundamental to such efforts, and that includes an investigating of renal fibroblasts, which are the cells that are primarily responsible for fibrogenesis.4-7 Fibroblasts were principal mediators of renal fibrosis, which contribute cellular elements being the sole source of ECM.2,5,8-10 Many studies have determined that the extent of interstitial involvement the accumulation of extracellular matrix (ECM) components including collagen types I, III, and IV, as well as proteoglycans and fibronectin.4,11-14 The fibroblasts in produce consequent to a fibronectin-rich and fibrillar ECM that fills up the interstitium, which leads, in turn, to nephron loss and the associated decline in kidney function. In this study, we investigation of the phenotypes of the interstitial fibroblasts, in addition to investigating the processes that may cause their modulation.
A number of growth factors have been found to be involved in the pathogenesis of renal interstitial fibrosis, including transforming growth factor (TGF-β1), which stimulates the deposition of both EMT and ECM.2,6,8,15 Many studies showed that TGF-β is major profibrotic cytokines and play a key role in fibrogenesis. TGF-β stimulate both type I and type II TGF-β receptors and upregulated the Smad family of transcriptional activators to transmit various signals. Such as receptor-regulated Smad (R-Smad), Smad2/3 are phosphorylated by activated type I receptors, which serves as a mediator for all of them.5,6,8,11,14,15 On the other hand, smad7 is firmly combined with TGF-β1 receptor, direct to the inability of smad 2/3 to be activated as well as inhibition of the signal transduction pathways. Smad7 inhibit the p-smad2/3, nuclear translocation of activated smad complexes, resulting in decreased fibronectin expression and complete inhibition of TGF-β signal transduction.2,5 When transcriptional activators are translocated into the nucleus, where they bind to specific Smad-binding elements in the regulate of TGF-β target genes and then effectively guide transactivation. TGF-β1/Smad signaling plays an important role in the ECM and, in turn, the pathogenesis of renal fibrosis. That said, further investigations are needed because the development of renal fibrosis is highly complex and our understanding of the condition remains far from complete.
Verbascoside can be found in species in Osmanthus spp .16 Verbascoside is hydrophilic in nature and relevant effects in the reduction of symptoms correlated to chronic pathologies and conditions such as Arthritis, Hypertension, Parkinson’s disease, Alzheimer’s disease, Estrogenic mediated diseases, Allergy type 1, Intestinal mucosytis.17-21 More importantly, Verbascoside also demonstrated various biological properties including anti-hepatotoxic, antinflammatory, anti-nociceptive, antioxidant and antihemolytic effects and antineoplastic properties in addition to numerous wound-healing and neuroprotective properties.17,19,20,22-23 However, literature no survey supporting the therapeutic effects of Verbascoside in treatment of renal fibrosis.
The present study was investigate the underlying mechanism of Verbascoside in renal cellular fibrosis, with the results showing that Verbascoside does, indeed, play a pivotal role in its regulation. The results further suggested that Verbascoside reversal of TGF-β1-induced cellular fibrosis while simultaneously suppressing the expression of fibronectin and fibrogenic signal proteins. More specifically, Verbascoside could potentially serve as an antagonist against both TGF-β1 signaling and fibrosis, in part through its down-regulation of TGF-β RII and pSmad2/3. In addition, Verbascoside increased negative-regulation of TGF-β proteins such as Smad 7 and E-cadherin. The findings of first evidence report that Verbascoside is a novel agent against TGF-b signaling and renal interstitial fibrosis.
Cell culture and treatment
NRK-49F cells (CRL-1570) were procured from the American Type Culture Collection (ATCC), while Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA) supplemented with 5% bovine calf serum (BCS), 100 U/ml penicillin, and 100 mg/ml streptomycin (HycloneLabs, Logan, UT) was used to culture a normal Rattus norvegicus kidney cell line at 37°C under 5% CO2. The cells were then trypsinized using 0.05% trypsin-EDTA (Hyclone).
Cell proliferation by MTT assay
MTT assays were performed to evaluate the cell viability of NRK-49F cells. Cells (1×104 cells/dl) were plated and incubated for 24 hrs in wells of a 96-well plate. Then treated with culture medium including on contraction of TGF-β (5ng/ml) and/or Verbascoside. After 24-hrs incubation, 10μl of sterile MTT dye were added, and the cells were incubated for 6 hrs at 37°C. Then, 100μl of acidic isopropanol (0.04 M HCl in isopropanol) were added and thoroughly mixed. Spectrometric absorbance at 595 nm (for formazan dye) was measured with the absorbance at 655 nm for reference.
LDH Assay for Cytotoxicity
Cells were maintained and passaged as described above. The cells were seeded in 96 well plates at a density of 2×104 cells/well in complete medium and incubated at 37oC in 5% CO2 overnight. Supernatant from the conditioned cells was collected and stored. Supernatant from maintained cells treated with 1% Triton X-100 was regarded as a positive control for maximum lactate dehydrogenase (LDH) release. After 24 h incubation at 37oC in 5% CO2, the supernatants were collected and centrifuged at 4,500g for 5 min to remove contaminating cells, and the level of LDH measured in duplicate using a cytotoxicity detection kit (Clontech , CA, US)
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was used to evaluate the expression of secreted fibronectin. To quantify fibronectin in the supernatant of cultured NRK-49F cells, conditioned culture medium was collected and centrifuged at 1200 rpm for 5 min to remove particulates. The clear supernatant was then collected and concentrated, then stored at −80°C for further use. A commercial sandwich enzyme-linked immunosorbent assay kit was used for detection of extracellular fibronectin (Takara Bio, Inc., Shiga, Japan) or TGF-β1 (R&D Systems, Minneapolis, MN). Detection was performed according to the manufacturer’s instructions. Sample absorbances at 450 nm were analyzed using an ELISA reader, and the concentration of each sample determined by interpolation with a standard curve, generated using an exogenous fibronectin (12.5, 25, 50, 100, 200, 400, 800 ng/ml) or TGF-β1 (0–2,000 pg/ml) as the standard.
Immunofluorescene Staining
Cells were cultured in glass chamber slides (Nunc, Rochester, NY) then fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS). After incubation blocked with 1% bovine serum albumin in PBS for 1 hr. The Cells were incubated with primary antibody overnight, washed with PBS, and incubated with the FITC-conjugated secondary antibody for 1 hr. The slides were mounted with Fluorescent Mounting Medium (Santa Cruz), and observed with a fluorescence microscope (Olympus, Japan).
Western Blot Analysis
Western blot assay was used to evaluate protein expression of the RII TGF-β receptors, and their downstream signal transducers (e.g., Smad7). In brief, cells were lysed using lysis buffer (10 mM Tris, 1 mM EDTA, 1% Triton X-100, 1 mM Na3VO4, 20 μg/ml aprotinin, 20 μg/ml leupeptin, 1 mM dithiothreitol, and 50 μM PMSF). The crude protein lysate was resolved by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Samples were then blocked with 10% (w/v) non-fat milk in Tris buffer saline tween (TBS-T) for 1h at 37°C. Individual membranes were probed with a 1 : 2000 (v/v) ratio of rabbit polyclonal antibodies to anti-Smad2/3 (sc-8332), anti-pSmad2/3 (sc-11769), anti-Smad7 (sc-11392), anti-E-cadherin (sc-7870), anti-TβRII (sc-1700; Santa Cruz Biotechnology, Santa Cruz, CA), and a 1 : 2000 ratio of anti-ß-actin (Sigma-Aldrich, St Louis, MO A-5316). After hybridization at 37°C, blots were washed and hybridized with 1:4000 (v/v) dilutions of goat anti-rabbit IgG or horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) or donkey anti-mouse IgG- or horseradish peroxidase (Santa Cruz). The signals were visualized using enhanced chemiluminescence (ECL), with β-actin as an internal control.
Statistics
The results were expressed as mean ± SEM. The unpaired Student’s t test was used for comparison between two groups. Values of P*<0.05 were considered to be statistically significant. For the in vivo experiments, a one-way analysis of variance (ANOVA) was used to determine if the means were significantly different (P<0.05). If the means were significantly different, a Tukey-Kramer multiple group comparison test was used to compare the individual groups. The standard error was indicated for each value by a bar, and the significance was indicated for each comparison. A one-way ANOVA test was used to calculate P values for all the ratios. All values were calculated using GraphPad Prism, version 3.00, for Macintosh (GraphPad Software, San Diego, CA).
We want to understanded the fibrosis-regulatory effects of Verbascoside extract on renal fibroblast cells. To examine whether Verbascoside blocks TGF-β-induced change of NRK-49F cells, we pretreated cells with TGF-β for 24 hours, followed by Verbascoside at the indicated concentrations for 24 hours. As Figure 1, cellular morphology was significantly and dose-dependently (i.e. 0.1, 1, 10 μM) restored compared with TGF-β- treating group (n = 4, p < 0.05).
We evaluate the underlying effects of Verbascoside on TGF-β -induced pharmacology. Cell survival and protein content were analyses in cells treated with TGF-β (5ng/ml) and Verbascoside (0.1, 1 and 10 μM) by LDH and MTT were performed (Figure 2A)(Figure 2B). We found that Verbascoside does not affect the viability of cultured fibroblasts. These observations demonstrated that either Verbascoside or TGF-β does not statistically affect cellular survival and protein content according to Figure 2. Thus, Verbascoside extract does not exert its effects by inhibiting cell growth or by inducing cell death.
To understanding the effects of anti-fibrosis on the regulation of TGF-β and Verbascoside dose-dependently in fibronectin bioactivity by ELISA asssy. TGF-β (5ng/ml) significantly increase extracellular fibronectin in NRK-49F cells compared to control in Figure 3A. More importantly, the Verbascoside dose-dependently (0.1, 1, 10 μM) and dramatically suppressed TGF-β-induced increases in secreted fibronectin levels. Example standard curve by the fibronectin ELISA kit (Figure 3A). Because TGF-β is a potent fibrogenic cytokine inducer for renal fibroblasts, it is argument to presume, that Verbascoside may contain certain active compounds with potential for fibrosis-inhibition.
To clarify the mechanism by which Verbascoside regulates renal cellular fibrosis, the expressions of TGF-β signal pathway were investigated. As shown in Figure 4, the administration of exogenous TGF-β (5 ng/ml) significant increases in the expression of fibronectin in the cells, whether of extracellular origin (as determined by ELISA) (Figure 3) or intracellular origin (as determined by Western blot) (Figure 4). More importantly, Verbascoside dose-dependently (0.1, 1, 10 μM) and dramatically suppressed TGF-β-induced increases in fibronectin levels. The presence of TGF-β receptors strongly correlates with susceptibility to cellular fibrosis. As shown in Figure 4, TGF-β induced a significant increase in the level of type II TGF-β receptors. Most importantly, Western blot analysis showed a statistically significant reduction expression of TβRII for the Verbascoside. Since the Smad family is the most important mediator for TGF-β signaling. As shown in Figure 4, TGF-β (5 ng/ml) significantly increased Smad2/3 levels. Intriguingly, Verbascoside dose-dependently (0.1, 1, 10 μM) dramatically suppressed TGF-β-induced increases in Smad2/3 in a dose-dependent manner. These observations show that Verbascoside may reverse TGF-β-induced cellular fibrosis by regulating and suppressing TGF-β down-stream signals. We examined Smad7 and E-cadherin, a powerful intracellular TGF-β antagonist. Treatment with TGF-β (5 ng/ml) induced significant decreases in Smad7 and E-cadherin. The Verbascoside dose-dependently (0.1, 1, 10 μM) and dramatically reversed TGF-β-induced decreases in Smad7 and E-cadherin. In other words, Verbascoside might ameliorate renal cellular fibrosis by inducing an increase in inhibitory Smad7 and E-cadherin. We found that 10 μM Verbascoside also dramatically decreased Smad 2/3. Thus, Verbascoside has potential to induce TGF-β signaling and so regulate renal cellular fibrosis.
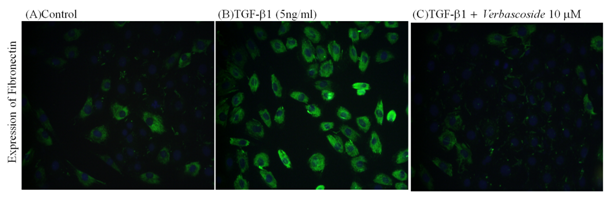
The morphologies of cells undergoing TGF-β induced fibroblast transition were observed through a immunoflourescence microscope. The expression of fibronectin of extracellular origin in the cells, however, was significantly increased by exogenous TGF-β1 as determined by immunoflourescence staining in Figure 5. Treatment with Verbascoside 10 μM restored the normal expression of the NRK-49F cells. On the basis of our results, we were able to identify a clear mechanism by which Verbascoside reverse renal cellular fibrosis. Specifically, the fibrosis appeared to be induced via the upregulation of the type II receptor of TGF-β1, as well as the upregulation of Smad 2/3 and fibronectin. At the same time, TGF-β1 caused a significant decrease in the expression of Smad7. Meanwhile, Verbascoside caused significant attenuation of the TGF-β1-induced upregulation of the type II receptor of TGF-β1, Smad2/3, as well as significant attenuation of the TGF-β1-induced downregulation of fibronectin. These results suggest that Verbascoside might be possible to downregulate TGF-β signal proteins, allowing it to be used as a novel treatment of renal fibrosis.
Our results demonstrate that Verbascoside plays a protective role against TGF-β1-induced renal fibrosis. Verbascoside antagonizes TGF-β1-induced renal fibrosis possibly by enhancing the expression of Smad7 in renal cells. Meanwhile, we show that Verbascoside increases the expression of E-cadherin. Thus, Verbascoside has the potential to inhibit TGF-β1-induced renal tubular fibrosis possibly by regulating Smads pathway. Due to the range of pharmacological activities and biological of Verbascoside and insufficient data on the safety reports it may be needed for assessing toxicity profiles. In this study, cellular toxicity of main constituent of Verbascoside was determined. We showed Verbascoside does not statistically affect cellular survival and viability according to Figure 2. These observations were consistent with those reported Aleo et al.33 Dell’Aquila et al.34 indicating revealed a significant antioxidant effect of Verbascosid .18,33,34. In 2013, Potapovich et al.35 showed Verbascoside offers additional skin-protection capacity against harmful ultraviolet radiation and inflammatory insults.35 Both of Georgiev et al.20 has been demonstrated HepG2 and NIH cells were exposed to different concentrations of Verbascoside in MTT assay.18 All of the above are consistent with this study.
When kidney injury occurs, activated renal cells can release TGF-β1 that in turn activates fibroblasts, therefore, TGF-β1 has a close relationship with fibrosis.6,9 The formation of renal fibrosis is a complex process of multifactor and multicell involvement. In this pathological process, the cell-cell, cell-cytokines and cell-matrix interactions constitute a cumbersome network. In this network, the development of renal fibrosis can be regulated by different signaling pathways and means. TGF-β1 is a major activator of kidney and a key mediator in the pathogenesis of renal fibrosis. TGF-β stimulates both type I and type II TGF-β receptors and then utilizes the Smad family of transcriptional activators to transmit various signals. Such as receptor-regulated Smad (R-Smad), Smad2, and Smad3 are phosphorylated by activated type I receptors, after which they bind to Smad4, which serves as a mediator for all of them.1,4,6,11,13,36 On the other hand, smad7 is firmly combined with TGF-β1 receptor, leading to the inability of smad 2/3 to be activated as well as inhibition of the signal transduction pathways. We speculate that Verbascoside may be used as a kind of treatment of renal fibrosis, and inhibit the formation of renal fibrosis.
In Figure 4, Verbascoside could be considered as an attractive therapeutic strategy for inhibition of cellular fibrosis after NRK-49F was stimulated with TGF-β1 in vitro. More specifically, Verbascoside can inhibit the expressions of TGF-β1/smad pathway in NRK-49F. Verbascoside can decrease smad2/3 expressions, and increase smad7 expression and increase E-cadherin expression suggesting that Verbascoside also plays a role in inhibiting NRK-49F activation and thus inhibit renal fibrosis, highlighting a potential anti-fibrotic mechanism, as illustrated in Figure 4. The findings of first evidence report that Verbascoside is a novel agent against TGF-β1 signaling and renal interstitial fibrosis. Reportedly, Verbascoside is regulated in different organ cells, including Verbascoside promoted apoptosis via p53 in human CRC cells.27 Furthermore reported that Verbascoside attenuates LPS-induced pro-inflammatory mediator production via TAK-1/JNK/AP-1 signalling in U937 cells.37 Therefore, the role of Verbascoside as a molecule able to regulated via different signal pathway in organ cells.
In summary, our study provides the new medicine Verbascoside, which exhibits potent effects in Verbascoside reverse renal fibrosis via TGF-β signal pathway. Thus, Verbascoside has potential to be developed into an therapeutic against renal fibrosis.
Figure 1 Effects of Verbascoside on the cell morphological transformations. The observation shows TGF-β1 induced NRK-49F cells fibrosis at different concentrations of Verbascoside to be treated by an inverted microscope. The experimental group of cells are without any morphological abnormalities, which represents Verbascoside does not affect the growth of NRK-49F cells. The experimental results showed that Verbascoside will not cause harm to the cells, and therefore does not have a cell cytotoxicity.
Figure 2 Effects of Verbascoside on levels of Cell proliferation by (A) LDH assay and (B) MTT assay. NRK-49F cells were treated with TGF-β (5ng/ml) in 0.5% BCS for 48 h, followed by treatment with Verbascoside (0.1, 1,10 µM) for a further 24 h. The absorbance of each sample was then analyzed by an ELISA reader. Supernatant was collected and subjected to MTT and LDH analysis. We found that Verbascoside does not affect the viability of cultured fibroblasts and inhibiting cell growth or inducing cell death.
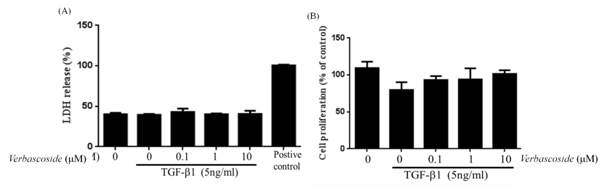
Figure 3 Effects of Verbascoside on levels of secreted fibronectin. The observation shows (A) Standard curves were generated using known concentrations of fibronectin (0,0.39, 1.56, 6.25,25 ng/ml). The absorbance (450 nm) of each sample was then analyzed by an ELISA reader. (B) NRK-49F cells were treated with TGFb1 (5ng/ml) in 0.5% BCS for 48 h, followed by treatment with Verbascoside for another 24 h. Supernatant was collected and subjected to fibronectin ELISA analysis. Fibronectin level was determined by interpolation with the standard curve. The fibronectin level of each condition was normalized to the cell number of each well. And contrast the normal control group, showing the amount of pathology group extracellular fibronectin has significantly increased, which means that changes in TGF-β1 can induce NRK-49F cells to secrete more fibronection. The observation ELISA showed that Verbascoside (10μM) dose-dependent reverse TGF-β1-induced increase in fibronection in NRK49F cells.
Figure 4 Effects of Verbascoside on TGF-β1-induced both of fibronectin expression and TGF-β1/Smads signaling pathway in NRK-49F cells. The observation shows NRK-49F cells were treated with TGF-β1 (5ng/ml) in 0.5% BCS for 48 h, followed by treatment with Verbascoside for another 24 h. Western blot analysis, Cell extracts were subjected to SDS-PAGE and immunoblot with a primary antibody against fibronectin. The expression of b-actin was used as an internal control. Western blotting showed that Verbascoside (10μM) dose-dependent reverse TGF-β1-induced increase in fibronectin expression and in NRK49F cells. And TGF-β1 through TGF-β1/Smads signaling pathway induced renal cellular fibrosis.
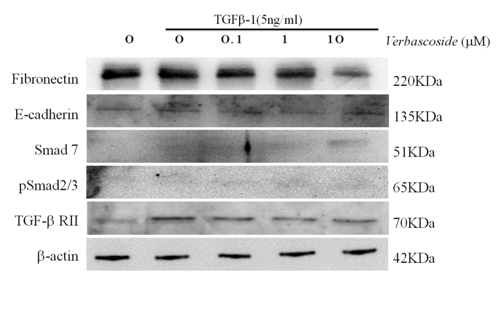
Figure 5 Effects of Verbascoside on TGF-β1-induced fibronectin expression in NRK-49F cells by Immunofluorescence assay. The observation shows NRK-49F cells were treated with TGF-β1 (5ng/ml) in 0.5 % BCS for 48 h, followed by treatment with Verbascoside for another 24 h. Immunofluorescence assy, grow cultured cells on chamber slides, fix cells by 4% Paraformaldehyde and add ice cold Triton X-100 break through the cells. At last immunoblot with a primary antibody against fibronectin. Immunofluorescence assay showed that Verbascoside (10μM) reverse TGF-β1-induced increase in fibronectin expression in NRK49F cells.
None.
Author declares that there is no conflict of interest.

©2016 Wang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.