Journal of
eISSN: 2374-6947


Research Article Volume 2 Issue 5
Medical Biochemistry Department, Tanta University, Egypt
Correspondence: Hala E Hamouda, Medical Biochemistry Department, Tanta University, Egypt Tanta Elstaad, Kamal Ghoraba street, Egypt
Received: May 17, 2015 | Published: December 8, 2015
Citation: El-Batch MM, Hamouda HE, Hassan SA, et al. Preliminary study of biochemical role of serum fetuin-a level and its gene polymorphism in diabetic patients with microalbuminuria. J Diabetes Metab Disord Control. 2015;2(5):158-164. DOI: 10.15406/jdmdc.2015.02.00053
Background: Microalbuminuria (MA) is a risk factor for cardiovascular disease. Fetuin-A is a hepatokines involved in insulin resistance (IR) and type 2diabetes. The aim of this study was to evaluate the possible association between both serum level and (Thr256Ser) gene polymorphism of fetuin- A and the pathogenesis of MA in type 2 diabetic patients, and determine its correlation with some vascular and oxidative stress markers.
Methods:50 patients with type 2 diabetes divided into two equal groups (normoalbuminuria and microalbuminuria groups) and 25 healthy subjects as controls. ELISA was used to measure serum fetuin-A and insulin levels, then assessment of IR. Fetuin- A gene (Thr256Ser) polymorphism was done using PCR-RFLP technique. Serum nitric oxide (NO) and ischemia modified albumin (IMA) were measured, in addition to routine investigations.
Results: Significant increase of serum IMA, NO, insulin and fetuin-A levels were documented in the microalbuminuric patient group as compared to the other two groups, with no statistically significant difference as regard the three fetuin-A genotypes either between both diabetic patient groups or between them and the control. By using multiple logistic regression analysis, Fetuin-A, is the most important predictor of MA.
Conclusion: So, it could be concluded that the higher levels of fetuin-A may be an independent novel risk factor for MA in type 2 diabetic patients, in spite of the non significant difference either between normoalbuminuric and microalbuminuric diabetic or between them and control according to the frequencies of the three fetuin-A genotype.
Keywords: diabetes mellitus, microalbuminuria, fetuin-a, no, IMA
MA, microalbuminuria; NO, nitric oxide; IMA, ischemia-modified albumin; IR, insulin resistance; UACR, urinary albumin/creatinine ratio; TG, triglycerides; TG, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostasis model assessment of IR
Microalbuminuria (MA) is the result of increased vascular permeability which develops as a renal manifestation of generalized vascular endothelial injury, that may make it a valuable early predictor of atherosclerosis and cardiovascular mortality, so the early identification and treatment of patients at increased risk for MA may be important to limit the excess renal and cardiovascular disease associated with type 2 diabetes.1 Therefore, more sensitive and specific biomarkers are required to predict the development of MA early enough to improve clinical management and to allow for timely intervention to prevent complications.2
Increased oxidative stress and reduced nitric oxide (NO) bioavailability play a causal role in endothelial cell dysfunction occurring in the vasculature of diabetic patients.3 In the kidney, NO controls both afferent and efferent vascular tone, the ultra filtration coefficient and medullary blood flow.4 Ischemia-Modified Albumin (IMA) is the variant form of human serum albumin of which N-terminal end has been altered after it was exposed to oxidative stress and/or ischemia, so it is considered as a novel marker for both tissue ischemia and oxidative stress.5
Fetuin-A is one of the most important hepatokines and is well known to be not only a powerful calcification inhibitor, but also, an endogeneous inhibitor of the insulin receptor tyrosine kinase in liver and skeletal muscle, resulting in insulin resistance (IR) in these target tissues.6,7 The human fetuin-A gene is located on chromosome 3q27, this segment of DNA contains genetic susceptibility loci for type 2 DM and metabolic syndrome.8
So, the aim of the study was to investigate the role of serum fetuin-A and its gene polymorphism in the pathogenesis of MA in type 2 diabetic patients by determining its relation with some vascular and oxidative stress markers.
The study population consisted of 50 patients, including 25 normoalbuminuric patients with type 2 diabetes (group II) and 25 microalbuminuric patients with type 2 diabetes (group III). Also, 25 matched healthy volunteers with no known chronic/systemic disorder and not on any medications were included to serve as healthy controls (group I). The control and patients were matched for age and gender.
The urinary albumin/creatinine ratio (UACR) in the morning spot urine collections was used to diagnose normoalbuminuria as < 30 mg/g and microalbuminuria as 30- 300 mg/g. The diabetic patients were recruited from the Outpatient Clinics of Endocrinology and Diabetes at Tanta University Hospital.
Subjects excluded from this study were those with other causes of MA including: acute febrile illness, liver dysfunction, congestive heart failure, malignancy, heart valve disorders, history of cerebrovascular or cardiovascular disorders, abnormal thyroid functions and other renal disorders. All subjects gave their written informed consent before participation. The study protocol was approved by the Local Ethics Committee of the Faculty of Medicine, Tanta University, and was in accordance with the principles of the Declaration of Helsinki II.
All the studied groups were subjected to the following
Routine laboratory investigations:
They included estimation of serum fasting and postprandial glucose, urea, creatinine, triglycerides (TG), total cholesterol (TC) and high-density lipoprotein (HDL) cholesterol using enzymatic colorimetric methods. Low-density lipoprotein (LDL) cholesterol was calculated by Friedewald’s formula.12
Estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) study equation: eGFR, in ml/min per1.73m2 = 175 × (serum creatinine, in mg/dl) −1.154 × (Age) −0.203 ×(0.742 if female) × (1.21 if black).13
Specific laboratory investigations including:
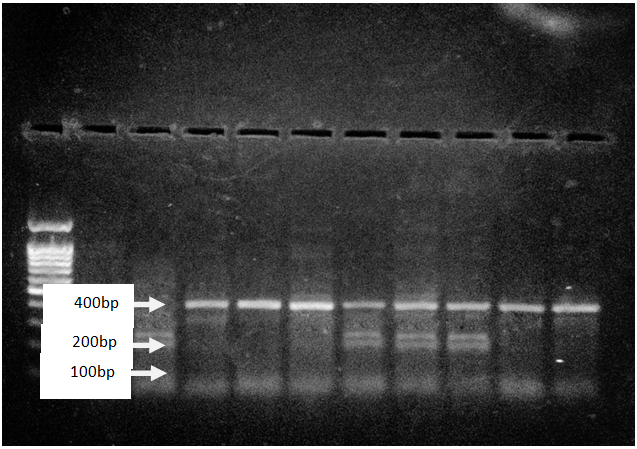
Above figure showed polymerase chain reaction (PCR) and restriction fragment length polymorphism analysis of fetuin-A gene (C→G); Thr256Ser polymorphism separated on an agarose gel.
Data were analyzed using SPSS software (version 11, SPSS Inc., Chicago, Illinois). Most data are expressed as mean ±standard deviation (±SD) for quantitative variables, whereas the frequencies of various alleles and genotypes were compared by Chi-square test (χ2). For each analysis, an odds ratio (OR) and 95% confidence interval (CI) were calculated. Comparison between groups was conducted using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Pearson’s correlation analysis was used to examine the relationships between the studied parameters. A p-value <0.05 was considered significant. To determine the associations of potential determinants with the development of MA, multiple logistic regression analysis was done, where the presence of MA was the dependent variable and potential determinants for development of MA were independent variables.
Demographic and clinical characteristics of all studied groups are presented in Table 1 in which increased disease duration was documented in microalbuminuric patients (group III) than normoalbuminuric patients (group II). Significant increase of blood pressure was detected in both diabetic groups as compared to control group. Laboratory characteristics of the studied groups are shown in Table 2. Significant increase of serum urea, creatinine, glucose, TC, TG and LDL-C levels were found in microalbuminuric patients as compared to both normoalbuminuric patients and controls, with significant decrease of serum HDL-C levels as compared to both groups. Normoalbuminuric patients showed significant higher levels of serum glucose, TC and LDL-C, as compared to controls with no significant difference between them as regard serum urea, creatinine, TG and HDL-C levels. Also, there were significant increase of serum insulin, IMA, NO and fetuin-A levels in addition to HOMA/IR in both diabetic groups as compared to controls, with significantly higher values in microalbuminuric patients as compared to normoalbuminuric patients. However, there was no significant difference as regard NO levels between the controls and normoalbuminuric patients.
Variables |
Group I |
Group II |
Group III(n=25) |
F/t/X2 |
P- value |
|
|
||||||
Age (years) |
49.5 ± 7.2 |
55.2 ±11.2 |
54.1± 7.5 |
F=2.95 |
0.06 ns |
|
Sex |
Female (%) |
21(84%) |
19(76 %) |
22(88 %) |
X2=1.29 |
0.53 (ns) |
Male (%) |
4(16%) |
6(24 %) |
3(12 %) |
|||
Duration (years) |
- |
5.5 ± 3.4 |
8.1 ± 4.6 |
t= -2.22 |
0.031 |
|
SBP(mmHg) |
114.4 ± 7.4 |
123.6 ± 7.8* |
121.2 ± 7.8* |
F=9.6 |
<0.001 |
|
DBP(mmHg) |
77.2 ± 4.1 |
82 ± 6.9* |
79.6 ± 7.9 |
F=3.4 |
0.039 |
|
Table 1 Demographic and clinical data of all of the studied groups.
Healthy Controls (Group I), Normoalbuminuric Type 2 DM (Group II), Microalbuminuric Type 2 DM (Group III), Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP)
ns: non-significant (P> 0.05) F: ANOVA test; X2: Chi square test, t: t-test. *significant as compared to Group I
Variables |
(Group I) (n=25) |
(Group II) (n=25) |
(Group III) (n=25) |
F |
P-value |
UACR (mg/g) |
16.7±9.3 |
18.6±5.9 |
73.5± 52*† |
27.5 |
<0.001 |
Urea (mg%) |
25.1±7.0 |
32.2±8.9 |
71.1±17*† |
109.9 |
<0.001 |
Creat. (mg%) |
0.7±0.1 |
0.8±0.2 |
0.9±0.3*† |
5.4 |
0.001 |
eGFR (ml/min per 1.73m2 ) |
92.2±16.7 |
94.3±28.8 |
70.4±24.9*† |
7.6 |
0.001 |
Fasting glucose (mg%) |
79.0±8.0 |
212.3±53.9* |
244.5±63.6*† |
82.3 |
<0.001 |
2-h PP blood glucose (mg%) |
93.8±16.6 |
278.1±59.9* |
356.3±52.4*† |
206.1 |
<0.001 |
TC (mg%) |
172.6±31.5 |
228.2±59.1* |
268.2±49.2*† |
25.03 |
<0.001 |
TG (mg%) |
95.9±14.2 |
114.4±40.5 |
136.7±34.7*† |
10.3 |
<0.001 |
HDL-C (mg%) |
49.7±4.4 |
46.9±9.5 |
38.8±5.8*† |
16.9 |
<0.001 |
LDL-C (mg%) |
104.3±29.5 |
158.4±61.5* |
202.1±48.2*† |
25.8 |
<0.001 |
Fasting insulin (μIU/ml) |
4.2±1.3 |
8.5±5.3* |
13.2±4.7*† |
29.4 |
<0.001 |
HOMA/IR |
1.1±0.3 |
4.6±3.6* |
7.8±4.3*† |
26.9 |
<0.001 |
IMA (ABSU) |
0.2±0.1 |
0.3±0.2* |
0.4±0.1*† |
11.9 |
<0.001 |
NO (μmol / L) |
8.7±3.7 |
14.3±8.9 |
32.7±19.4*† |
25.2 |
<0.001 |
Fetuin-A (mg%) |
26.2±2.8 |
31.6±11* |
39.6±7.7*†+ |
17.9 |
<0.001 |
Table 2 Biochemical findings among all the studied groups
Healthy controls (Group I), Normoalbuminuric type 2 DM (Group II), Microalbuminuric type 2 DM (Group III), Urinary Albumin To Creatinine Ratio (UACR), Estimated Glomerular Filtration Rate (egfr) , 2 Hour Post Prandial (2-H PP) Blood Glucose, Total Cholesterol (TC), Triacylglycerol (TG),High Density Lipoprotein Cholesterol (HDL-C), Low Density Lipoprotein Cholesterol (LDL-C), Homeostasis Model Assessment Of Insulin Resistance (HOMA-IR), Ischemia Modified Albumin (IMA), Nitric Oxide (NO),
ns: non-significant (P> 0.05) *significant as compared to Group I; † significant as compared to Group II
In Table 3, significant correlation was found between UACR and all the studied parameters except age, fasting blood glucose and TC, in both diabetic patient groups (n= 50), with the highest correlation found with HOMA/IR, serum insulin and fetuin-A levels. In multiple logistic regression analysis considering microalbuminuria is the outcome variable. It was found that fetuin-A followed by NO, TG and fasting insulin levels were the most important predictors of MA (Table 4). By using Receiver Operating Characteristics (ROC) curve for fetuin-A (at cutoff value > 26.5 mg%), HOMA/IR (at cutoff value > 5.86), NO (at cutoff value> 16.9 µmol/L), IMA (at cutoff value > 0.24 ABSU) in group II versus group III, it was found that fetuin-A had the highest sensitivity, negative predictive value and accuracy (96, 94.4, and 0.8 respectively) for detecting MA (data not shown) .
Parameters |
UACR (mg/g) |
|
|---|---|---|
R |
P-value |
|
Age (years) |
0.05 |
0.8 |
Duration (years) |
0.36 |
0.01* |
Urea (mg%) |
0.56 |
<0.001* |
Creatinine (mg%) |
0.52 |
<0.001* |
eGFR (ml/min per 1.73m2 ) |
-0.56 |
<0.001* |
Fasting blood glucose (mg%) |
0.14 |
0.3 |
2h-PP blood glucose (mg%) |
0.34 |
0.02* |
TC (mg%) |
0.17 |
0.2 |
TAG (mg%) |
0.72 |
<0.001* |
HDL-C (mg%) |
-0.37 |
0.007* |
LDL-C (mg%) |
0.32 |
0.025* |
Fasting insulin (μIU/ml) |
0.70 |
<0.001* |
HOMA/IR |
0.77 |
<0.001* |
NO (μmol / L) |
0.53 |
<0.001* |
IMA (ABSU) |
0.37 |
0.007* |
Fetuin-A (mg%) |
0.54 |
<0.001* |
Table 3 Person’s correlation between UACR and other studied parameters in diabetic patient groups (group I, group II) (n =50)
Variables |
B |
S.E. |
Wald |
P-value |
OR |
95.0% C.I. for odd |
|
|---|---|---|---|---|---|---|---|
Lower |
Upper |
||||||
TG |
-0.091 |
0.036 |
6.370 |
0.012* |
0.913 |
0.851 |
0.980 |
NO |
0.253 |
0.091 |
7.737 |
0.005* |
1.288 |
1.078 |
1.539 |
IMA |
1.666 |
2.901 |
0.330 |
0.566 |
5.289 |
0.018 |
1559.516 |
Fasting insulin |
0.987 |
0.435 |
5.141 |
0.023* |
2.683 |
1.143 |
6.295 |
HOMA/IR |
-0.677 |
0.447 |
2.291 |
0.130 |
0.508 |
0.212 |
1.221 |
Fetuin-A |
0.155 |
0.103 |
8.250 |
0.001* |
5.454 |
3.954 |
15.429 |
Constant |
-6.550 |
3.896 |
2.827 |
0.093 |
0.001 |
|
|
Table 4 Multiple logistic regression analysis using 6 biologically important variables as independent variables, and MA as the dependent variable
B: Regression Coefficient, S.E: Standard Error, OR: Odds Ratio CI, Confidence Interval
As regards the results of genotyping of fetuin-A gene polymorphisms, no significant differences between either the two diabetic groups [with chi-square(X2) =0.09, odds ratio=1.2, 95% confidence interval; 0.4-3.9, p value =0.8] (data not shown) or between both of them and control group (with X2=1.13, p value =0.9) according to frequencies of the different genotypes and alleles (Table 5). These data could indicate the weak relationship between the risky genotypes CG and GG and either development of diabetes or MA. It was observed that patients with the risky genotypes CG and GG had higher levels of TC, IMA and fasting insulin levels, but lower levels of HDL-C, when compared with CC genotype (data not shown).
|
Group I |
Group II |
Group III |
Total |
Chi-square |
|
|---|---|---|---|---|---|---|
N (%) |
N (%) |
N (%) |
N (%) |
X2 |
P-value |
|
CC |
17(68) |
17 (68) |
16 (64) |
50 (66) |
1.13 |
0.9 (ns) |
CG |
8 (32) |
7 (28) |
8 (32) |
23 (30) |
||
GG |
0 (0) |
1 (4) |
1 (4) |
2 (2.67) |
||
C allele |
42 (84) |
41 (82) |
40 (80) |
123 (82) |
0.27 |
0.9 (ns) |
G allele |
8 (16) |
9 (18) |
10 (20) |
27 (18) |
||
Table 5 Comparison among the studied groups according to the frequency of fetuin –A genotype polymorphism (C→G); Thr256Ser, using Chi-square test
ns: non significant (P>0.05)
The present study documented that fetuin-A level was an independent predictor for microalbuminuria, supported by the multiple logistic regression analysis. Serum fetuin-A levels showed significant increase in both patient groups as compared to controls with significant higher levels in microalbuminuric patients as compared to normoalbuminuric.
These results came in agreement with Huddam et al.19 who found higher serum fetuin-A levels in metabolic syndrome patients with MA. Also, Inoue et al.20 has documented that the higher urinary excretion of fetuin-A is a risk factor for both MA and reduction of GFR in DN in addition it may reflect an increased in its serum levels. In contrast, Ramadan et al.21 demonstrated an association between lower fetuin-A levels and microvascular complications in type 2 diabetic patients with early DN. Also, Jung et al.22 revealed that serum fetuin-A level was not significantly different according to the absence or the presence of diabetic micro angiopathies.
Fetuin-A has been known as a natural inhibitor of tyrosine kinase activity of insulin receptor. Previous epidemiological studies showed an independent association between fetuin-A and insulin resistance and future development of diabetes mellitus in general population.23-25
In the present study, fasting serum insulin levels and HOMA/IR index, the indicators of insulin resistance in type 2 diabetes, were significantly increased in both patient groups with significantly higher values in microalbuminuric patients. These findings are in agreement with the results of Esteghamati et al.26 and Hsu et al.27 Also, our data showed significant positive correlation between serum fetuin-A and both fasting serum insulin and HOMA-IR supporting the hypothesis that fetuin-A is involved in the pathophysiology of insulin resistance.
Therefore, it is possible that the role of fetuin-A in mediating insulin resistance may underlie the association between fetuin-A and microalbuminuria. Compensatory hyperinsulinaemia, characterizing IR, could lead to endothelial dysfunction by increasing the availability of endothelin-1, decreased NO availability and by changes in intracellular calcium and magnesium metabolism. Also, hyperinsulinaemia may increase sodium lithium counter-transport activity and stimulate renal sodium reabsorption leading to volume expansion, increased adrenergic activity and hypertension.26
Furthermore, impaired insulin sensitivity is associated with mesangial hyperplasia, and renal hypertrophy and increased endothelial cell proliferation and lipid and hyaluronate deposition in the renal matrix and inner medulla, effects that may directly contribute to progressive kidney damage.28 In addition, Ix and Sharma29 demonstrated that higher fetuin-A levels lead to suppression of adiponectin transcription in adipocytes and lower adiponectin levels reduce 5’-AMP activated protein kinase in podocytes to promote podocyte foot process effacement and albuminuria.
Other potential mechanism could explain the association between high fetuin-A levels and microalbuminuria which is the lipid profile abnormalities observed in our microalbuminuric patient that can mediate endothelial dysfunction and future cardiovascular diseases,30 supported by significant positive correlation found between serum fetuin-A level and both TAG, LDL-C levels in the current study.
It that been revealed that fetuin-A directly or indirectly leads to these changes through its inhibitory effects on the insulin receptor tyrosine kinase. Inhibition of the insulin receptor by fetuin-A may lead to increased lipolysis and efflux of free fatty acids from adipose tissue. This may, in turn, lead to increased production of apolipoprotein B–containing very-low-density lipoprotein (VLDL). Furthermore, hypertriglyceridemia may lead to a decrease in the cholesterol content of HDL enhancing HDL clearance from the circulation, thereby potentially leading to the atherogenic lipid profile as such observed in this study.31 The infiltration of atherogenic lipoproteins into the glomerular endothelium and mesangial cells can initiate a cascade of events, including adhesion molecule expression, monocyte chemo attractant production, and release of reactive oxygen species, that lead to early glomerular injury.32
Therefore, the possible explanation for our findings is the role of fetuin-A in mediating insulin resistance, lipid profile abnormalities and endothelial dysfunction which underlie the association between fetuin-A and abnormal albuminuria. Since it has been demonstrated that at least four non synonymous polymorphisms exist in the fetuin- A gene (at amino acid positions Thr248Met [C→T], Thr256Ser [C→G], Asp276Asn [G→A], and Arg317Cys [C→T]), genetic polymorphisms may have an effect on circulating levels of this protein.33
The most known allelic variant in clinical studies is the (Thr256Ser) which has been associated with serum fetuin-A levels,34 type 2 diabetes35 and ischemic stroke.8 Therefore, this study aimed to examine the relationship between this common allelic variant (Thr256Ser) for fetuin-A gene and the risk of developing microalbuminuria in type 2 diabetic patients to aid in its early diagnosis. However, our data do not provide evidence for a significant association between the studied Thr256Ser polymorphism of the fetuin-A gene and neither serum fetuin-A levels nor prognostic effect for the progression to microalbuminuria in this population.
These results came in agreement with the findings of Zeidan et al.18 and Maharem et al.36 who conducted their study on Egyptian chronic kidney disease patients (CKD) and found no statistically significant difference between CKD patients and control groups regarding the frequencies of the three fetuin-A genotypes (C→G), and no significant difference was found between fetuin-A genotypes as regards vascular calcification and atherosclerosis assessed by measurement of intima-media thickness. However, Ma et al.8 found that frequencies of the GG genotype and the G allele in fetuin-A (rs4918) were significantly higher in patients with ischemic stroke or atherosclerotic cerebral infarction than those in the control group. The lack of association with serum fetuin-A levels in this study may be explained by reduced sample size that does not provide adequate statistical power to detect genetic associations of modest effect. Also, it may be attributed to the different populations. Moreover, it seems possible that the use of diabetes medications by our study participants could influence fetuin-A levels, which need further investigations.
Although the underlying mechanisms of endothelial dysfunction and kidney damage in type 2 DM are multifactorial, substantially evidence suggests that increased reactive oxygen species (ROS) may be a cause.37 In the present study, significant higher levels of both NO and IMA in microalbuminuric diabetic patients as compared to normoalbuminuric diabetic patients and controls were detected, which came in accordance with several studies,38-40 but in contrast to others.41,42
Prolonged exposure of endothelial cells to high glucose increases both NO and superoxide anion production. It enhances NO synthesis by endothelial cell NO synthase (eNOS) in afferent arterioles and glomerular endothelial cells leading to preferential dilatation of afferent arterioles, which ultimately induces glomerular enlargement and glomerular hyper filtration, which in turn leads to an increase in urinary albumin excretion and thus causes progression of nephropathy in early type 2diabetes.43
Also, the observed high levels of IMA here-in may be due to local overproduction of ROS in the diabetic kidney or the altered catabolism of modified albumin in the kidney which supports the involvement of oxidative stress and ischemic-hypoxia in pathogenesis of diabetic complications.40
Ukinc et al.44 in their study stated that elevated IMA in diabetic patients is due to uncontrolled oxidative stress occurs on endothelium due to hyperglycemia and presence of other conventional risk factors and the effects of subsequently released reactive oxygen types on albumin. Since IMA levels were high in patients with MA and it was positively associated with hsCRP and MA levels, they think that they may accept IMA as a novel risk factor and a marker in diabetic patients for extensive endothelial dysfunction, low grade inflammation, and macrovascular disease that may develop in the future. In the view of all of these outcomes, IMA can be an additional parameter to show sub-clinic vascular disease.
There is several weakness of the study;
High level of fetuin-A, which is the most important predictors of MA, may lead to endothelial dysfunction in systemic and renal blood vessels and predispose these patients to increased risks of both MA and cardiovascular disease, through its effect on either lipid profile, IR or endothelial dysfunction but, with lack of the effect of its risky rare genotypes (CG, GG). So fetuin-A could be a potential novel target for not only the treatment and/or prevention of human IR in type 2 diabetes mellitus patients, but also of its vascular complications including MA. Our study supported the involvement of oxidative stress in pathogenesis of diabetic nephropathy and IMA may be a helpful clinical marker in estimating kidney dysfunction in diabetic patients. Also, the microalbuminuric DN stage would be more suitable for antioxidative intervention.
None.
Author declares that there is no conflict of interest.

©2015 El-Batch, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.