Journal of
eISSN: 2374-6947


Review Article Volume 1 Issue 3
Department of Metabolism and Cardiovascular Disease, State University of Rio de Janeiro, Brazil
Correspondence: Vanessa Souza Mello, Department of Metabolism and Cardiovascular Disease, State University of Rio de Janeiro, Av 28 de Setembro 87 fds. 20551-030 Rio de Janeiro, RJ, Brazil, Tel +55.21.2868 8689, Fax 2868 8033
Received: July 04, 2014 | Published: July 16, 2014
Citation: Schultz A, Carlo Magliano DA, Bringhenti I, et al. Nonalcoholic steatohepatitis: lessons from different diet-induced animal models. J Diabetes Metab Disord Control. 2014;1(3):59-67. DOI: 10.15406/jdmdc.2014.01.00014
The nonalcoholic fatty liver disease has been considered the hepatic manifestation of the metabolic syndrome, which is prone to progress to nonalcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma. Modern lifestyle, which includes less time dedicated to exercise parallel to preference for fast foods rich in fats and simple carbohydrate, has been suggested as the cornerstone for the development of metabolic impairments linked to obesity. In this way, experimental models are useful in trying to mimic metabolic pathways and morphological alterations involved with the spectrum of nonalcoholic steatohepatitis pathophysiology. Diets rich in saturated fatty acids, trans fatty acids and fructose emerge as the most relevant dietary models to induce nonalcoholic steatohepatitis. Consideration should be given to the composition of the diet and time of administration, both of which can induce many different results. Pharmacological approach aims to alleviate insulin resistance, lipotoxicity and inflammation, which are pivotal to NASH triggering. The resulting control of the underlying metabolic impairments reduces NASH expressively. All in all, the jury is still out on the adequate management of this disease. Giving that dietary patterns exert a crucial role in NASH progression, considerations should be given to the exact mechanisms by which each nutrient underpin NASH development.
Keywords: nonalcoholic steatohepatitis, fructose, saturated fatty acids, trans fatty acids, sucrose, treatment, insulin resistance, inflammation
ACC, acetyl-coa carboxylase; ACL - ATP-citrate lyase; ACOX1, acyl coa oxidase; ALIOS, american lifestyle induced obesity syndrome; ALT, alanine transaminase; ARB, angiotensin receptor blocker; AT1R, angiotensin ii type 1 receptor; ChREBP, carbohydrate responsive element binding protein; CPT1a, carnitine palmitoyl transferase 1A; DM2, type 2 diabetes mellitus; DNL, De novo lipogenesis; ER, endoplasmic reticulum; FAS, fatty acid synthase; FFAs, free fatty acids; GLUT 2, glucose transporter 2; HFCS, high fructose corn syrup; HFG, hepatocyte growth factor; HFHSD, high-fat and high-sucrose diet; HSCs, stellate cells; ICAM-1, inter cellular adhesion molecules; IKKß, ikb kinase ß; IL-6, inter leukin-6; IR, insulin resistance; IRS-1, insulin receptor; MAPK, Mitogen Activated Protein kinases; MCD, methionine and choline-deficient diet; MUFA, mono unsaturated fatty acid; NAFLD, non alcoholic fatty liver disease; NASH, non alcoholic steato hepatitis; PPAR, peroxisome proliferator activated receptor; PUFA, poly unsaturated fatty acid; RAS, renin angiotensin system; SCD1, stearoyl coenzyme a desaturase-1; SFA, saturated fatty acid; SREBP-1, sterol regulatory element binding proteins 1c; TGF-ß, transforming growth factor-beta1; TNF-a, tumoral necrosis factor alpha; TRANS, trans fatty acids; UCP-2, un coupling protein 2; WAT, white adipose tissue; a-SMA, alpha smooth muscle action
Currently, NAFLD (nonalcoholic fatty liver disease) is considered the hepatic manifestation of the metabolic syndrome and may progress to NASH (nonalcoholic steatohepatitis), cirrhosis and hepatocellular carcinoma.1 Although NAFLD and NASH are associated together, and the pathogenesis of NASH is not yet completely elucidated. The pathogenesis of NASH was considered as the "First hit", characterized by the accumulation of intrahepatic lipid due to disrupting metabolic pathways and increase vulnerability to cell damage. Lipotoxicity triggers the "Second hit", which encompasses increased oxidative stress that triggers the secretion of tumoral necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), activation of hepatic stellate cells (HSCs) and expression of cellular adhesion molecules (ICAM-1, Intercellular adhesion molecules; E-selection or P-selection).2,3 The lipotoxicity, mitochondrial dysfunction and magnitude of oxidative stress result in lipid peroxidation, which leads to death of hepatocytes, inflammation and ultimately, fibrosis.4
Changes in lifestyle as dietary interventions or prescriptions of exercise, as well as the pharmacological treatments have not been established. As a rule, the progression NAFLD -NASH is related to the continuity of the stimulus that generates the injury, i.e.: chronic dietary fat intake.5 These observations highlight a need to exactly understand how different nutrients underpin hepatic alterations that can result into NASH and to establish suitable approaches to tackle this metabolic constraint.
Dietary models of NASH are relevant as an attempt to mimic the pathogenesis of the current epidemics of diet-induced obesity and the resulting metabolic disturbances, which includes NAFLD and NASH.1
In general, animals fed diets rich in lipogenic nutrients, particularly simple sugars such as fructose or sucrose6,7 and/or saturated fats,7?10 develop steatosis rather than steatohepatitis. In older animals, some evidence of hepatocellular injury, focal lobular inflammation and even early pericellular fibrosis may be observed, particularly during prolonged intake of a lipogenic diet.7,8,11 Other commonly used fatty liver disease models are animals lacking genes that affect appetite regulation, or which predispose to diabetes.12?16
High-fat diet and NASH
High-fat feeding when used as a model for NASH produces variable results with respect to the levels of steatosis, inflammation and fibrosis that is dependent on the rodent species, fat content, duration of treatment and qualitative composition of dietary fat.17
Nowadays, the “two-hit” hypothesis is widely accepted as the pathogenesis of NAFLD/NASH, however, an obligatory role for oxidative stress in the progression to NASH could not been shown.18
As an alternative hypothesis, emerging evidence now points to metabolites of fatty acids as the real culprits in the hepatocellular injury in NASH, as they are identified in other target organs of lipotoxicity. Lipotoxicity is the phenomenon that refers to the cellular dysfunction seen when accumulation of excessive lipid occurs in non-adipose tissue such as the liver.19
The literature has shown that both quantity and quality of dietary fat influence liver fatty acid synthesis,20 insulin sensitivity and can induce IR (insulin resistance).21 Among high dietary fat models, intragastric overfeeding of mice seems to resemble the histopathology and pathophysiology of human NASH most closely. This model, however, requires technical expertise and specialized equipment that may hamper its widespread use.
More recent studies prefer to use overfeeding because livers of the overfed mice were metabolically characterized by an increased expression of (pericentrally located) lipogenic genes (FAS, Fatty acid synthase; SCD1, Stearoyl-Coenzyme A desaturase-1; and PPAR-γ, Peroxisome proliferator-activated receptor y), combined with a high portal inflow of dietary free fatty acids (FFAs). These nutritional and metabolic sources of fat explain the initial homogeneous lipid accumulation observed in the livers of these mice. At later stages, the basal expression of lipolytic genes (CPT1a, carnitine palmitoyl transferase 1A; ACOX1, acyl CoA oxidase; and PPAR-α, Peroxisome proliferator-activated receptor α), combined with the maximal inflow of dietary FFAs cause the observed periportal lipid accumulation on top of the initially homogeneous steatosis.20–23
In an attempt to create an experimental model to study NASH, it was used a liquid high fat diet given ad libitum to Sprague-Dawley rats or a controlled daily fat intake by force-feeding Sprague-Dawley rats.24 In these two cases, high fat diet induced mild steatosis and huge hepatic inflammation. The main fat component of these two diets was corn oil, consisting of 13% (w/w) saturated fatty acid (SFA), 24% monounsaturated fatty acid (MUFA) and 59% polyunsaturated fatty acid (PUFA). These PUFA were almost entirely composed by pro-inflammatory n-6 polyunsaturated fatty acids which are known to be involved in liver oxidative stress.25 This rat model is suitable for diet-induced obesity.
The intragastric overfeeding of C57BL/6 mice with a high-fat diet (37% of corn oil in the diet, corresponding to 185% normal energy intake) over 9 weeks to induce NASH in male C57BL/6 mice provoked overweight (71% higher body weight), with increased visceral fat (white adipose tissue [WAT]), hyperglycemia, hyper insulinemia, hyper leptinemia, glucose intolerance and IR. Almost half of the animals (46%) developed NASH with a 5- to 6-fold increase in plasma alanine transaminase (ALT). However, these models represent excessive overfeeding and some of the biochemical changes observed in the liver did not mimic those seen in NASH patients.22 Furthermore, the human NASH features usually have a diet with higher levels of SFA and cholesterol and low levels of PUFA.26 It is noteworthy that C57BL/6 is an inbred mouse model that provides suitable background for studying NASH as whenever fed onto high fat diet, they manifest early metabolic syndrome features.27
The effects of giving a high-fat diet to rodents can be highly variable. Some of this variability could be explained by the influence of rodent strain, which is known to be important in the susceptibility to several types of liver disease. In a longitudinal study, chronic administration of a high-fat diet (60% of calories from fat) led to the development of steatohepatitis in male C57BL/6 mice, after 50weeks.28,29 Likewise, a high-fat diet (60% lard) has been used to trigger the “second hit” and to initiate steatohepatitis in fa/fa (defective long-form Leptin receptor) rats.30 In this model, oxidative stress was proposed to initiate steatohepatitis, because there was an increase in NADPH oxidase activity, lipid peroxidation and protein carbonyl formation together with decreases in GSH and antioxidant enzymes, such as catalase. Similarly, a high-fat diet administered to foz/foz mice with a dysfunctional Alstrom syndrome 1 (ALMS1) gene induced steatohepatitis in these obese animals.31
A murine model incorporating prolonged administration of a “western diet” with high saturated fat and cholesterol content was able to reproduce NASH with some increase in fibrosis markers, but not ballooning.32
It’s well described the proatherogenic effect of the trans fatty acids. However, little is known about the influence of trans fatty acids on hepatic lipid metabolism. A recent study evaluated the consumption of 3 different high-fat diets (PUFA, SFA and Trans Fatty acids (TRANS)) in male LDLr-KO mice (LDL Receptor Knock-Out mice) and demonstrated that the mice fed the TRANS diet had greater hepatomegaly due to fat accumulation and inflammatory NASH-like lesions and impaired glucose tolerance when compared with mice fed the PUFA and SFA diets.33 In agreement with another study, the TRANS diet intake elicited increased transcription of genes involved in liver fat synthesis such as PPAR-γ and Sterol regulatory element-binding proteins 1c (SREBP-1c) and was associated with higher hepatic cholesterol and TG concentrations compared with the PUFA and SFA diets.34 Recently, LDLr-KO mice have been described as a suitable model to detect the onset inflammation in NAFLD, which is crucial to NASH development.35
Another study observed that NASH was induced by the American lifestyle induced obesity syndrome (ALIOS diet) model for an initial period of 16weeks. The ALIOS model includes feeding male C57BL/6 mice with high fat, trans-fat enriched chow and consumption of high fructose corn syrup resulting in a NASH-like liver histological phenotype.36
Fructose and NASH
The consumption of sugar incorporated in the diet increased their rates by 3 times in the last 50 years in a global context.37 The corn syrup, high fructose (HFCS) is a sweetener produced by the isomerization of existing glucose fructose corn syrup. HFC-90 (90% 9% fructose and glucose) is the main product of such chemical reactions and is diluted with glucose syrup to form HFCS - and HFCS 42-55, these are the most commonly used in processed foods and beverages as soft drinks, fruit and bread to yogurts.38 The low cost and easy handling pointed to the main attractions for increased consumption.39,40
In 1986, the average intake of fructose has increased 16% from 56g/day to 65g/day in 2007.41 The 74g/day consumption, equivalent to 2.5 cans of soda affect a rise in blood pressure without cardiovascular risk.42
Along with the increasing growth of the amount of fructose in the diet, an increase in the presence of obesity, IR and hypertension in the United States, leading producer of high-fructose syrup from corn.37 Studies report that more than 10% of daily calories come from fructose. Ingestion of a sweetening agent in the diet is 75% in adults and 82% children.43
Glucose transporter 2 (GLUT2) mediates the entry of fructose in the liver and frutokinase rapidly phosphorylates it into fructose-1P. Fructose-1P is converted into dihydroxyacetone and glyceraldehyde-3P. The latter will have different targets:
In hepatic lipogenesis, lipid oxidation is inhibited by fructose, favoring the formation of fatty acids linked to glycerol, triglycerides and VLDL. Despite being the smallest amount of fructose converted into fatty acid, chronic intake pre-offers the framework of hepatic steatosis. Increased DNL associated with the process of dyslipidemia and hepatic steatosis, mechanisms promoted by fructose.44 The continuous production of acetyl-CoA due to mobilization of fructose exceeds the responsiveness of mitochondria (Krebs cycle) and this excess is converted to citrate, leaving the cytosol and being the substrate for DNL. In addition, acetyl-CoA form malonyl-CoA (reaction mediated ACC - acetyl-CoA carboxylase) inhibits the action of CPT-1a, decreasing the input of fatty acids into the mitochondria for oxidation.45 On the other hand, fructose directly or indirectly activates transcription factors (SREBP-1c and Carbohydrate responsive element-binding protein - ChREBP) responsible for the activation of genes encoding enzymes of DNL (ACL, ATP-citrate lyase; ACC and FAS), leading to production of VLDL and triglycerides. All factors combined culminate with the storage of fat in the form of macro and micro vesicles within hepatocytes.44
For the analysis of NAFLD and NASH, it is not conclusive to say that the effects of chronic ingestion of fructose develop these valences. Various parameters such as dose-response, time and delivery vehicle are offered from many experimental studies.46
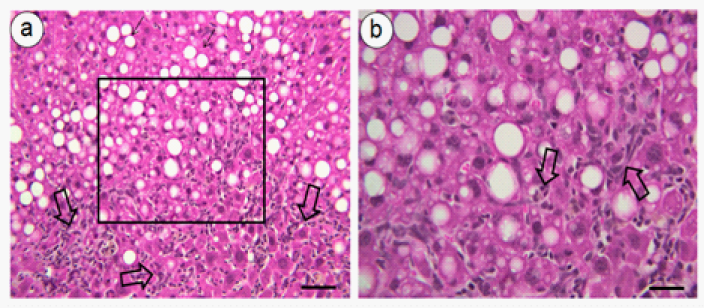
The onset of hepatic steatosis frames for possible progression to NASH, mediated by obesity; these lipogenic effects are not dependent on the increase of body mass.47 Sprague Dawley rats fed a 60% fructose for 28days showed increased levels of mRNA factors transition of hepatic lipogenesis (ACC, FAS, SREBP-1c and ChREBP) without change in body mass. On the other hand, Wistar rats fed with 70% fructose for 5weeks showed large amount of macro and micro vesicles of fat and intra hepatic intralobular inflammation.8 C57BL/6 mice consuming a diet rich in fat and carbohydrate intake associated with the 55% fructose in water for 16weeks developed significant fibrosis with NASH and obesity.7 We observe livers from C57BL/6 mice subjected to 34% fructose or fructose combined with a high-fat diet (34% fructose and 42% lipid - lard) for 16weeks, showing inflammatory infiltrate [depicted in Figure 1a-1b].48 The results show that mice fed a high-fructose diet are good models for the development of NASH.49,50

Sucrose and NASH
Studies in animals have found that high carbohydrate diet induced histopathological features of the NAFLD and NASH, especially when administered at high doses.6?8,51?53 Some studies have shown the induction of NAFLD and NASH by high-sucrose diet, alone or in interaction with other diets. In this model, hepatic steatosis occurs first and steatohepatitis develops later. On the other hand, in a dietary model induced by methionine and choline deficiency, steatohepatitis occurs very quickly.54?57 Discrepancy between studies may be partially a result of differences in the experimental diet and its time administration period.
The metabolic pathways accomplishing carbohydrate conversion into fat are well known and they were well characterized in earlier studies.58,59 In animal models, numerous studies have addressed the effects of diets enriched with fructose or sucrose. Several studies using animal models have shown that a high-sucrose diet leads to hepatic steatosis. Wistar rats fed a high-sucrose diet showed an induction of multiple components of the unfolded protein response in the liver consistent with endoplasmic reticulum (ER) stress, and hepatic lipid accumulation, liver injury and inflammation.60 In other study, male C57BL/6 mice fed a high-carbohydrate diet (65% sucrose) for long-term (16weeks) induced typical hepatic steatosis and, for the feeding period employed, the liver did not display markers of hepatitis (in?ammation, necrosis and others).6 Whether feeding this diet for longer periods may lead to NASH remains to be established.
Current evidence shows that sucrose diet altered the liver structure. C57BL/6 mice fed to 32% sucrose for 8weeks showing abundant micro- and macrovesicular steatosis with areas of inflammatory infiltrate (Figure 2).61
Interestingly, adult male Sprague-Dawley rats fed with high-sucrose diet (70% sucrose) for 2 or 3weeks developed fatty livers and became obese, being the fat distributed periportally and the absolute weights of the livers and liver/body weight ratio increased by 20%,7 where as Wistar rats fed with a sucrose rich diet (70% sucrose) for 5weeks demonstrated mild steatosis, inflammation and lipogranulomas in the liver tissues, without steatohepatitis.8 Moreover, C57BL/6 mice fed a 65% sucrose diet for 8weeks exhibit obesity, IR and macrovesicular steatosis.62
Experimental studies seldom identify potential genes/pathways that may contribute to the progression of liver steatosis to NASH. Enzymes involved into glucose and fructose metabolism and their conversion to fat59,63 were up regulated by the high-sucrose diet model. In addition, the transcription factor SREBP-1c, which controls the transcription of genes involved in fatty acid synthesis,64 and whose transcription is regulated by insulin,65 were up regulated.
The metabolism of sucrose-rich diets generates fructose, which exerts acute effects on hepatocyte energy homeostasis.11 Thus, pathology that occurs during the chronic ingestion of high-sucrose diets is caused by fructose. The phosphorylation of fructose causes an abrupt decline in hepatocyte ATP content that gradually recovers as phosphofructose and is metabolized via the pentose phosphate shunt. The activity of this metabolic pathway requires a sufficient pool of diet that causes purine depletion and exacerbates the fatty liver that is caused by high-fructose diets in rats.11,66
Mixed models
Diets that are enriched with a high-sucrose/fructose diet develop steatosis in Fischer 344 rats.67 In addition, Zucker fatty (fa/fa) rats are also particularly sensitive to fatty livers induced by feeding 60% sucrose or orotic acid (1%).68 In all strains of rodents that have been evaluated, males are more susceptible to fatty liver than females.
Emerging data indicate that specific dietary nutrients, as sucrose, when coupled with methionine and choline deprivation, can influence MCD-mediated liver disease.52 Recent study reported a diet model of NASH by using a MCD diet associated a sucrose diet, in which C3H/HeJmice developed hepatic steatosis, hepatocellular apoptosis, alanine aminotransferase elevation, lipid peroxidation and hepatic inflammation.52 Others studies have shown that mice feeding of high-sucrose (54%) diets that are also deficient in choline (0.05%) for 6months showed perivenous fatty change and hepatic fibrosis in most of the rats.11 Deficiency of methionine and choline, which are essential for hepatic β-oxidation and production of VLDL, enhances the lipogenic effect of high sucrose diet.54
Excessive sucrose and long-chain saturated fatty acids in the diet of Zucker rats and C57BL/6 may play a role in the development and progression of NAFLD.53,69,70 High-fat and/or high sucrose diet induced steatosis in C57BL/6 mice leads to hepatic cell depletion, a similar Th-1 polarization to that in ob/ob mice and exaggerated lipopolysaccharide sensitivity.69 Recent studies demonstrated that ingestion of a diet rich in fat and sucrose by C57BL/6 mice significantly elevated WAT weights, hepatic steatosis and plasma insulin levels as early as 2weeks.71 In addition, rodents exposed to high-fat and high-sucrose diet (HFHSD) for 2weeks demonstrate fat accumulation in the liver, however without histopathological features of NASH.70
Methionine- and choline-deficient-diet
The methionine- and choline-deficient-diet (MCD) is frequently utilized to establish NASH in the rodent liver. Rodents fed MCD-diet develop steatohepatitis producing changes on the redox balance and hepatic lesions that mimic the impairment of patients with NASH.72,73 It happens because the choline and the methionine amino acids are essentials for hepatic β-oxidation and production of VLDL.51 Choline is an essential nutrient that participates in cell membrane integrity, phosphatidylcholine synthesis, transmembrane signaling, neurotransmission and methyl metabolism. Dietary with choline deficiency promotes hepatic steatosis and reduces plasma VLDL level as well established in the literature. It was thought that these effects were caused due to impaired synthesis of phosphatidylcholine, which could diminish VLDL synthesis and secretion, but observations74 have brought doubts about it and suggest other mechanisms and further studies to define the role of choline deficiency in steatosis.54 Furthermore, the lack of the methionine, an essential nutrient as well as choline, increases oxidative stress, impairs phosphatidylethanolamine synthesis and the transport of fat from the liver to extrahepatic sites causing hepatic steatosis.72 It fits highlight that mice fed a diet that is deficient in both choline and methionine develop inflammation and hepatic fibrosis in addition to steatosis.54
Serum alanine aminotransferase level is consistently increased after MCD-diet administration, steatohepatitis in this diet-model occurs at day 10, and perisinusoidal fibrosis is observed by 8-10weeks in mice. After 10weeks of MCD-diet administration, it can be observed extensive macrovesicular steatosis in all areas except in the periportal region and many necroinflammatory foci containing lymphocytes, and neutrophils are observed in mice.50 Nevertheless, it is important to note that although the MCD-diet causes these effects, NASH in rodents fed MCD-diet may depends on the species, gender and strain of the animal.75 A study76 has compared the effects of MCD-diet using male and female rats (Wistar, Long-Evans and Sprague-Dawley) and C57Bl/6 mice. It was found that male Wistar strain had the greatest NASH rate of all rats groups, but C57Bl/6 mice developed the most expressive necrosis and inflammation, in addition to the best histological features of NASH.
Evidences suggest that MCD-diet leads to induction of alcohol-inducible CYP2E1 expression that also is observed on patients with NASH. These findings support the hypothesis that alcoholic and nonalcoholic steatohepatitis could share the same pathogenic mechanisms.77,78 CYP2E1 is a member of the cytochrome P450 mixed-function oxidase system and can produce ROS by mitochondria. These ROS, in turn, cause peroxidation of membrane lipids resulting in alteration of the membrane function. Moreover, products of peroxidation of lipids can react with functional groups of amino acids of proteins and enzymes, altering their function, as in uncoupling protein-2 (UCP-2) and CPT-1.73,79
By decreasing oxidative defense mechanisms, MCD-diet creates a situation that is known to induce TNF-alpha and others proinflammatory cytokines. Oxidants and TNF-alpha are also expected to activate the IkB kinase β (IKKβ) pathway that interacts with others pathway signaling as insulin and renin angiotensin system (RAS). However and interestingly, this NASH model did not develop the metabolic profile observed in typical patients with NASH. Animals fed the MCD-diet are cachectic and show significant weight loss (often, more than 20% weight loss after three weeks and 50% compared with control mice by 10weeks), low fasting glucose, serum insulin, Leptin and triglycerides levels and peripheral insulin sensitivity. An unchanged or an increased Adiponectin level in plasma is also observed.80?82 Moreover, the histological distribution of hepatic steatosis differs from the pattern seen in humans where a periportal rather than perivenous deposition is noted.66
Taking all these information into account, we can highlight two main conclusions: first, MCD-diet is an excellent model to induce steatohepatitis in mice models rather than in rats models, even some changes in Wistar rat being observed; and second, MCD-diet reproduces the inflammatory and lipid profiles of NASH, including activation of RAS and HSCs, but differently of human NASH, this diet did not reproduce the metabolic profile, induces a significant weight loss and present some difference between loci of collagen deposition in liver. To improve the metabolic problems, it is frequent use some genetically obese mice, such as ob/ob and db/db mice. Finally, the main advantage of the MCD-diet is that it is easy to obtain and use.50
Drug treatment to NASH should target metabolic alterations that underpinned NAFLD and inflammation.83,84 Insulin sensitizers have become the main approach, given that IR is closely linked to hepatic steatosis development.85 More recently, an important role in the pathogenesis of NAFLD was attributed to angiotensin II and the use of angiotensin receptor blockers (ARBs) to tackle NASH has been put forward. Hypolipidemic agents such as fibrates and statins have also been addressed owing to the prevailing involvement of lipoprotein metabolism disturbances into hepatic lipotoxicity.86
Insulin sensitizers
Metformin: Metformin has been the first option to treat type 2 diabetes mellitus (DM2) since 1994 when it was approved by Food and drug administration. It is a biguanide, causing reduction of hepatic glucose production, which in turn, alleviates hepatic IR. In humans, Metformin seems to be efficient in reducing hepatic steatosis parallel to decreased inflammation and fibrosis.85,87
Concerning experimental data, administration of 0.1% of Metformin during 8weeks provoked substantial reduction of hepatic triglyceride, reversed hepatic steatosis in histological evaluation, suppressed HSCs activation, reduced the transcription of genes involved with lipogenesis, inflammation and fibrogenesis in mice fed MCD+HFD.88
Thiazolidinediones: PPAR-g agonist, such as Pioglitazone, is widely used to counter IR and promote diabetes control.89 PPAR-gamma is a transcription factor that regulates gene expression in many tissues, mainly in liver and white adipose tissue, being crucial to hepatic lipogenesis and adipogenesis, respectively. Besides, therapy with PPAR-gamma agonist has been linked with inhibition of cell proliferation and collagen expression in HSCs in vitro and in vivo,90,91 making PPAR-gamma agonists a potential approach to treat NASH.
Pioglitazone (1mg/Kg day) prevented rats with NASH due to MCD diet from developing cirrhosis. A significant amelioration of liver histology, followed by decreased expression of alpha smooth muscle actin (α-SMA), transforming growth factor-beta1 (TGF-β), procollagen and TNF-α was observed.92 All of these endpoints highlight efficiency in tackling inflammation and fibrosis prevention.
Higher levels of circulating adiponectin, which enhances insulin sensitivity and ameliorates NASH, have been reported in rodents under Rosiglitazone treatment.93,94 In Sprague-Dawley rats with NASH induced by high-fat high-cholesterol diet, treatment with 4mg/Kg day of Rosiglitazone yielded modulation of adiponectin receptor in liver (decrease) and white adipose tissue (increase). Reduced circulating levels of TNF-α also contributed to tackle NASH as it is inversely correlated with adiponectin levels.95,96
Other agents: Glucagon like peptide-1 analog also exhibits positive effects, minimizing body mass, liver mass, liver steatosis and fibrosis in ob/ob mice with NASH induced by high trans- fat diet.97 Sitagliptin (dipeptidyl peptidase-4 inhibitor) reduced steatosis, ameliorated hepatic ultra structure by enhancing beta-oxidation and reducing lipogenesis in the liver of C57BL/6 mice fed with a high saturated-fat diet, preventing from NASH development.98
Angiotensin Receptor Blockers (ARB): RAS activation is involved in liver fibrosis due to activation of HSCs, which mainly express angiotensin II type 1 receptor (AT1R). By blockade of AT1R, Telmisartan inhibits HSCs activation by angiotensin II, reducing liver fibrosis (111,162). Furthermore, Telmisartan acts as partial PPAR-g agonist and, hence, has been indicated as the first-class option for patients with NASH.86
In the MCD diet model, Telmisartan markedly alleviated hepatic steatosis, inflammation, and fibrosis in Fischer rats when compared to Valsartan (pure ARB). Conversely, Pioglitazone (total PPAR-gamma agonist) reduced NAFLD, but did not decrease subcutaneous and visceral fat like Telmisartan did.99 Then, dual ARB/PPAR-gamma agonist appears as the most promising approach to tackle NASH.
Due to selective PPAR-gamma activation, Telmisartan ameliorates IR and promotes ectopic fat redistribution into proliferating adipocytes, reducing glucolipotoxicity.100,101 In C57BL/6 mice fed MCDHF diet, the treatment with telmisartan (10mg/Kg day) had striking effects upon liver steatosis, hepatic triglycerids and fibrogenesis. Treated animals showed rise in adiponectinemia, reduced size of adipocytes, suppression of macrophage infiltration into liver, decreased expression of mRNA for type 1 collagen and TGFβ1, implying inhibition of fibrogenesis and prevention of hepatocellular carcinoma.100
Likewise, a study pursued in rats fed chronically with MCD diet showed that the daily dose of 3mg/kg of Telmisartan has the potential to improve NASH possibly owing to increased hepatocyte growth factor (HGF) production. This observation emphasizes the partial PPAR-gamma agonist property of Telmisartan, making this drug differs and be more attractive than others ARBs.102 Even with diagnosis of cirrhosis after 24weeks feeding rats with MCD diet, Telmisartan at the dose of 2mg/Kg day avoided hepatocellular carcinoma occurrence.103 After all, Telmisartan has been reported as a suitable strategy to prevent NAFLD from becoming NASH in different diet-induced models.98,102
Fibrates
PPAR-alpha is essential to the b-oxidation process within hepatocytes, which counteracts lipogenesis making output of FFAs surpasses their input, alleviating hepatic steatosis. For that reason, Fenofibrate (pure PPAR-alpha agonist) has been used to treat NASH.104,105 In APOE2 knock-in mice fed with a western diet, Fenofibrate treatment decreased hepatic macrophage accumulation, which preceded hepatic steatosis and abolished hepatic lipotoxicity. This observation was accounted for by massive reduction in the expression of inflammatory genes and increased expression in b-oxidation related genes, all of which were PPAR-alpha target genes. In addition, procollagen type 1 expression was diminished by fibrate.106,107
Bezafibrate (50 or 100mg/Kg day), a pan-PPAR agonist, and GW501516 (10mg/Kg day), a PPAR-delta agonist, inhibited the MCD-diet-induced NASH. Both of them increased the levels of hepatic mRNAs linked to Beta-oxidation and lipid transportation within hepatocytes concomitant with reduction in the levels of mRNA associated with inflammatory cytokines. Bezafibrate also enhanced levels of adiponectin and its receptors 1 and 2, ameliorating IR, which has a pivotal role in liver lipotoxicity.80
Other drug classes
Nifedipine, a calcium channel blocker, activates PPAR-gamma. Hence, it targets IR and inflammation, two paramount features in NASH pathogenesis. In a rat model of NASH due to MCD diet, Nifedipine effects bore resemblance to Bezafibrate treatment, emerging as a promising option to treat NASH in hypertensive individuals.108
Statins are the most prescribed drug to treat dyslipidemia worldwide. Nearly 70% of patients with NASH have dyslipidemia, which justify its evaluation in NASH. Rosuvastatin has striking effects upon diet induced NAFLD in C57BL/6, favoring b-oxidation instead of lipogenesis and blocking inflammatory pathways.109 In humans, preliminary studies indicate favorable effects of treatment with Atrovastatin and Rosuvastatin in patients with NASH.110,111 However, results seem to be inconclusive, for instance, simvastatin did not show positive effects upon NASH.112 Works that are more detailed should be carried out to unravel the benefits and safety of using statins to control NASH.
Overall, taking into account the largely controversial clinical data and the vast amount of options to treat NASH indicated by experimental studies, attention should be drawn to high cost and known side effects of pharmacological agents while choosing. (Figure 3) summarizes the main effects of the drugs proposed to treat NASH.
Taken together, the animal studies described above clearly configure excellent models to induce steatohepatitis, mainly in mice and rats. These experimental models are useful in trying to mimic metabolic pathways and morphological alterations involved with the spectrum of NASH pathophysiology in humans. Diets rich in nutrients like SFA, Trans fatty acids, fructose, sucrose or even the absent of others (like methionine and choline) may disrupt different pathways, yielding nonalcoholic steatohepatitis. Consideration should also be given to the time of administration, which can induce many different results. Since NAFLD is considered the hepatic manifestation of MS and may progress to NASH, it is essential to understand the molecular mechanisms involved in the development of this disease. In this way, pharmacological approach aims to alleviate IR, lipotoxicity and inflammation, which are pivotal to NASH triggering. All efforts in trying to treat or avoid NASH are relevant, given that it represents greater risk to Hepatocellular carcinoma and cirrhosis, both of which exhibit higher lethality and can be induced by dietary inadequacies facing the recent nutritional transition and global obesity pandemic.
None.
Author declares that there is no conflict of interest.

©2014 Schultz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.