Journal of
eISSN: 2374-6947


Research Article Volume 5 Issue 2
1Department of Pharmacology, Sinhgad Dental College & Hospital, India
2Department of Pharmacology, Pathology, MGM Medical College, India
3Department of Pharmacology, Hind Institute of Medical Sciences, India
Correspondence: Rajesh Kumar Suman, Assistant professor Department of pharmacology, Hind Institute of medical sciences, Mau, Ataria, Sitapur, Lucknow, India,
Received: January 21, 2018 | Published: March 28, 2018
Citation: Borde MK, Mohanty IR, Maheshwari U, et al. Natural dipeptidyl peptidase-4 inhibitor Terminalia arjuna mitigates myocardial infarction co-existing with diabetes in experimental rats. J Diabetes Metab Disord Control. 2018;5(2):48–56. DOI: 10.15406/jdmdc.2018.05.00137
Background: Large pool of diabetic patients have co-existing cardiovascular diseases, DPP-4 inhibitors may represent novel and promising ant diabetic agents with potential cardiovascular benefits. However they are expensive drugs and recently have been associated with a number of unacceptable adverse effects.Terminalia arjuna have been reported to possess ant diabetic activity, its cardioprotective effects in the setting of diabetes has not been studied so far. DPP-4 based therapeutics may represent novel anti-diabetic drug, the cardioprotective actions of which may translate into demonstrable therapeutic benefits in diabetes with co-existing cardiovascular diseases. With this point of view the present study was designed.
Material and methods: The study is experimental study conducted in department of pharmacology, MGM Medical College, Navi Mumbai. The rats were randomly allocated in various group for 5 week. STZ 45 mg/kg administered on 1st week to induce the diabetes and Isoproterenol 85 mg/kg administered on 2 subsequent days before scarification of rats to induce myocardial infarction. Blood glucose >200 mg/dl on end of the 1st week confirm the diabetes. Hydoalcoholic extract of Terminalia arjuna was fed orally from 2nd week to 5th week and rats were scarified for biochemical and histopathological parameter.
Results: Efficacy of Terminalia arjuna (TA) was observed on various parameter of diabetes with cardiovascular complication. TA treatment showed restoration of body weight as compare with control group. The treatment with TA reduced hyperglycemia as compare with (Diabetes control group) D-ISP group. Vildagliptin (VIL) treatment showed superior effect in controlling hyperglycemia as compare with TA. The VIL & TA treatment group rats showed significantly reduced heart to body weight ratio as compared to D-ISP rats. VIL & TA treated group significantly reversed the STZ/ISP induced increase in CPK-MB, hs-CRP levels and marked protection against cardiac damage was observed as indicated by decrease in serum CPK-MB isoenzyme, hs-CRP in treated rats as compared to D-ISP group rats. VIL & TA treated rats showed significant reduction in serum DPP-4 levels as compared to D-ISP rats. Significant cardioprotection as indicated by positive correlation between cardiac marker CPK-MB and serum DPP-4 in VIL (r = 0.899; p < 0.01), TA (r = 0.848; p < 0.05) groups was also confirmed by histopathological assessment. As per histopathological and biochemical report, TA treatment does not show delineated effect in pancreas, liver and kidney.
Conclusion: Terminalia arjuna demonstrated beneficial effects in experimental model of myocardial infarction co-existing with diabetes.
Diabetes mellitus (DM) and ensuing cardiovascular (CV) complications have arisen as the epidemic of the early 21st century. Diabetes mellitus remains a profound risk factor for cardiovascular disease. Excess mortality in type 2 DM is largely related to an increased incidence of CV disease with approximately 75% of deaths in people with diabetes attributable to stroke, myocardial infarction (MI) and peripheral arterial disease.1,2 Management of cardiovascular risk is an essential aspect of diabetes care and acceptable CV risk is a requirement for Antidiabetes medications. DPP-4 inhibitors are a novel class of oral hypoglycemic agents, widely used for the treatment of type 2 diabetes mellitus (T2DM). Besides established ant diabetic effects, several studies have reported the cardioprotective benefits of DPP-4 inhibitors via GLP-1 dependent and independent pathways. Studies documented that, DPP-4 inhibitors improve several cardiovascular risk factors: they improve glucose control (by reducing the risk of post prandial and fasting hyperglycemia), weight neutral, lower blood pressure, improve dyslipemia, reduce inflammatory markers, diminish oxidative stress, improve endothelial functions and reduce platelet aggregation in patients with T2DM.3,4 In Zucker diabetic fatty rats, a genetic rodent model for type 2 diabetes, the inhibition of DPP-4 corrected glycemic dysmetabolism, hypertriglyceridemia, inflammation and hypertension.5 Since a large pool of diabetic patients have co-existing cardiovascular diseases, DPP-4 inhibitors may represent novel and promising ant diabetic agents with potential cardiovascular benefits. Thus, DPP-4 inhibitors are a promising new therapeutic approach for the management of type 2 diabetes. However they are expensive drugs and recently have been associated with a number of unacceptable adverse effects.6–9 In this scenario, if novel DPP-4 inhibitors from alternative sources are developed, that share the advantages of DPP-4 inhibition but at the same time overcome the limitations of the currently available synthetic DPP-4 inhibitors, it would be very beneficial. Terminalia arjuna (Combretaceae), commonly known as Arjuna, has been used in Indian system of medicine for over three centuries. The thick, white to pinkish gray bark has a long ethnomedicinal history including cancer treatment; cardioprotective, hypotensive, hypolipidemic and wound healing activity.10 Medicinal plants have been observed to possess numerous activities with regard to hypoglycemic and cardiovascular disorder. Despite the wide usage of medicinal herbs for several pathological conditions in Ayurveda, few systemically designed studies are available to validate their therapeutic effects. Although Terminalia arjuna have been reported to possess antidiabetic activity,11–13 its cardioprotective effects in the setting of diabetes has not been studied so far. DPP-4 based therapeutics may represent novel anti-diabetic drug, the cardioprotective actions of which may translate into demonstrable therapeutic benefits in diabetes with co-existing cardiovascular diseases. With this point of view the present study was designed. The results of present study will provide preliminary experimental evidence on the potential therapeutic benefits of using natural DPP-4 inhibitors in the setting of diabetes co-existing with cardiovascular diseases. Various parameters like anti-diabetic, cardioprotective, anti-inflammatory, antioxidant, DPP-4 pathway, safety {pancreas, liver and kidney function} and histopathological indices of injury were evaluated in experimental groups.
Chemicals and test drugs
Streptozotocin (STZ) and Isoproterenol (ISP) were procured from Sigma Chemicals St Louis, USA. The test drugs Vildagliptin was obtained as gift sample. The hydro-alcoholic dried standardized extract of Terminalia arjuna were procured from Sanat Pharmaceutical, New Delhi. All other chemicals and reagents used were of analytical grade.
Experimental animals
The study has been conducted at MGM medical college and hospital Navi Mumbai. Wistar rats were procured from Bombay Veterinary College, BVC Campus Road, Parel, Mumbai. Adult male Wistar rats, 10 to 12 weeks old, weighing 150 to 200 gm were used in the study. Rats were housed in the Animal Facility of Mahatma Gandhi Mission Medical College, Navi Mumbai, Indiain polyacrylic cages (38x23x10cm) under standard laboratory conditions. The animals were allowed free excess to standard diet, tap water adlibitum and allowed to acclimatize for one week before the experiments. The study protocol was approved by the Institutional Animal Ethics Committee and conforms to the Committee for the Purpose of Control and Supervision of Experiments on Animals and Indian National Science Academy and Guidelines for the Use and Care of Experimental Animals in Research.
Experimental model of myocardial infarction in the setting of diabetes mellitus
Male wistar rats weighing 150- 200 gm was used for the study. Type II Diabetes was induced in rats by a single Streptozotocin injection (45 mg/kg body wt, intraperitoneal {i.p.} dissolved in 0.01 M cold citrate buffer, pH 4.5) in overnight fasted rats. Serum glucose estimations (blood sugar > 200 mg/dL) were undertaken periodically (day 0, 3 and 7) from the tail vein to confirm the production of diabetes mellitus. Animals showing fasting blood glucose higher than 200 mg/dL were considered as diabetic. Myocardial infarction (MI) was produced by Isoproterenol injection (85 mg/kg body weight, subcutaneous {s.c.} injection dissolved in normal saline) 24 and 48 h prior to scarification (5th week). At the end of experimental period, rats were sacrificed for further biochemical investigations as well as histopathological evaluation.
Experimental groups and treatment protocol
Group 1: Normal control (NC): In Normal Control group, rats were administered distilled water per orally using a feeding cannula for study period of 5 weeks.
Group 2: Diabetic ISP control (D-ISP): The rats were administered distilled water per orally using a feeding cannula for study period of 5 weeks. Streptozotocin (45 mg/kg body weight, i.p.) was injected to induce diabetes at 0 week and challenged with Isoproterenol (85 mg/kg body weight sc.) 24 and 48 h prior to scarification.
Group 3: Vildagliptin (VIL): Vildagliptin (10 mg/kg) was administered orally using a feeding cannula from 1st to 5th week (4 weeks). The Streptozotocin (45 mg/kg body weight, i.p.) was injected to induce diabetes at 0 week. Subsequently the rats were challenged with Isoproterenol (85 mg/kg body weight sc) 24 and 48 h prior to sacrification.
Group 4: Terminalia arjuna (TA): Terminalia arjuna (500 mg/kg) was administered orally using a feeding cannula from1st to 5th week (4 weeks). The Streptozotocin (45 mg/kg body weight, i.p.) was injected to induce diabetes at 0 week. Subsequently the rats were challenged with Isoproterenol (85 mg/kg body weight sc) 24 and 48 h prior to scarification.
Assessment of body weight changes
Each rat was weighed individually twice, first at the beginning of the experiment (initial weight) and second, 24 h after the administration of the last dose of either drug (final weight). The difference in body weight of each rat was calculated and expressed as percentage change according to the following:
% change in body weight = Final weight –Initial weight X 100/Initial weight
Biochemical Parameters
Biochemical parameters were estimated using AU 480 auto-analyzer Backman coulter, Germany. The rat blood samples of all experimental groups were collected from the retro-orbital plexus under light ketamine anesthesia (40 mg/kg i.p.) at 0, 1st and 3rd week for estimation of blood glucose and creatinine phosphokinase (CPK-MB). In addition, after the completion of the experimental duration (5th weeks), Serum was used for the determination of the biochemical parameters blood glucose,14 HbA1c,15 creatinine phosphokinase (CPK-MB),16 serum DPP-4,17 high sensitive C-reactive protein (hs-CRP),18 pancreatic lipase,19 serum glutamate pyruvate transaminase (SGPT),20 creatinine21 by AU480 auto analyzer Backman coulter, Germany or ELISA kits in the Pathology (NABL accredited) and Pharmacology laboratory.
Histopathological studies
At the end of the experiment (5 weeks), the animals were sacrificed. The heart, pancreas, liver and kidney were immediately fixed in 10% buffered neutral formalin solution. The tissues were carefully embedded in molten paraffin with the help of metallic blocks, covered with flexible plastic moulds and kept under freezing plates to allow the paraffin to solidify. Cross sections (5 mm thick) of the fixed tissues were cut. These sections were stained with hematoxyline and eosin and visualized under light microscope to study the microscopic architecture of the tissues. The investigator performing the histological evaluation was blind to biochemical results and to treatment allocation. (H&E 40×)
Statistical analysis
All numerical data in text, figures and tables were expressed as the mean + SD. Statistical analysis was performed by student t test and One-way analysis of variance (ANOVA). Spearman correlation coefficient was used to determine the relationship between different variables Differences were considered statistically significant at p<0.05.
General observations and assessment of body weight changes
Body weight of rats in all the groups was recorded every week and the percentage change in body weight was calculated after 5th week. The percentage change in body weight in NC group rats at the end of 5th week was found to be 14.45, D-ISP;-10.03, VIL;-1.78, TA; 4.97. The D-ISP group rats showed significant decrease in body weight (%) as compared with NC, VIL, TA treated groups. TA treatment showed significant (p<0.01) restoration in body weight as compared with VIL group (Figure 1).
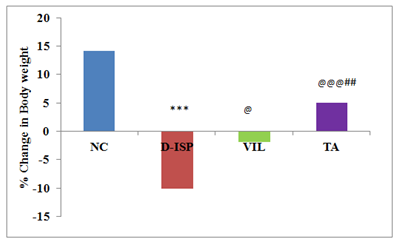
Figure 1 Body weight changes among various experimental groups.
Values are expressed as mean±S D.
***p<0.001NC VS D-ISP; @@@p<0.001,@ p<0.05 D-ISP VS VIL, TA; ##p<0.01 TA VS VIL.
NC, Normal control (n=8); D-ISP, Diabetic-isoproterenol control (n=9); VIL, Vildagliptin (n=8); TA, Terminalia arjuna (n=7)
Comparative cardioprotective efficacy of natural and synthetic DPP-4 inhibitors
Diabetic parameter: Hyperglycemia induced by Streptozotocin was maintained throughout the study period as evidenced by persistent hyperglycemia throughout the study duration. There was a significant (p<0.001) increase in blood glucose and glycosylated hemoglobin levels in D-ISP group rats as compared to NC group. Oral feeding of TA (500 mg/kg) significantly restored (p<0.001) the elevated blood glucose levels as compared to D-ISP group rats. Similarly glycosylated hemoglobin was also reduced in TA (500 mg/kg) treatment group as compared to D-ISP group rats. As shown in figure, VIL (10 mg/kg) as compared to D-ISP group was significantly decreased the high glucose and glycosylated hemoglobin followed by TA (500 mg/kg) treatment. The anti diabetic efficacy of VIL was found to be superior to TA therapy (Figure 2) (Figure 3).
Cardiac parameters: The D-ISP control rats showed significantly increase in (p<0.001) heart to body weight ratio as compared to NC rats. The VIL (10 mg/kg) (p<0.01) & TA (500 mg/kg) (p<0.05) treatment group rats showed significantly reduced heart to body weight ratio as compared to D-ISP rats. VIL (10 mg/kg) treatment significantly reduced (p<0.05) heart to body weight ratio as compared to TA (500 mg/kg) (Figure 4). There was a significant increase in cardiac markers of injury CPK-MB (p<0.001), hs-CRP (p<0.01) level in D-ISP rats as compared to NC group at 5th week of study after ISP challenge. VIL (10 mg/kg) & TA (500 mg/kg) treated group significantly reversed the STZ/ISP induced increase in CPK-MB (p<0.001), hs-CRP (p<0.05) levels at 5th week. A marked protection against cardiac damage was observed as indicated by decrease in serum CPK-MB isoenzyme, hs-CRP in treated rats as compared to D-ISP group rats. However, the cardioprotective efficacy of the marketed synthetic DPP-4 inhibitor: Vildagliptin was found to be superior to TA (p<0.01) (Table 1). The serum DPP-4 levels (p<0.001) increased significantly in D-ISP group rats as compared to NC group rats. VIL (10 mg/kg) & TA (500 mg/kg) treated rats showed significant reduction in serum DPP-4 levels as compared to D-ISP rats. The VIL (10 mg/kg) treated rats showed significant reduction (p<0.01) in serum DPP-4 levels as compared to TA (500 mg/kg) treated rats. Significant cardioprotection as indicated by positive correlation between cardiac marker CPK-MB and serum DPP-4 in VIL (r = 0.899; p < 0.01), TA (r = 0.848; p < 0.05) groups was also confirmed by histopathological assessment (Table 1), (Figure 5) (Figure 6).
SN |
Parameters |
Variable |
NC |
D-ISP |
VIL |
TA |
1 |
Cardiac parameters |
CPK-MB (U/L) |
1565.12+292.07 |
5424.28+837.73*** |
2311.25+253.96@@@## |
2865.71+119.7@@@ |
2 |
hs-CRP(mg/dl) |
0.86+0.11 |
1.9 +0.5** |
0.91+0.1@@# |
1.2+ 0.22@ |
|
3 |
DPP-4 Pathway |
Serum DPP-4 (microunit/ml) |
4.76+0.43 |
38.25+4.25*** |
12.22+1.35@@@# # |
18.42+ 2.04@@@ |
Table 1 Cardiac parameters and serum DPP-4 levels among various experimental groups
Values are expressed as mean±SD.
***p<0.001 NC VS D-ISP;
@@@ p<0.001;
@@ p<0.01,
@ p<0.05D-ISP VS VIL,TA;
## p< 0.01,#<0.05 VIL VS TA.
CPK-MB, Creatinine phosphokinase-MB; hs-CRP, High sensitive reactive protein; DPP-4, Dipeptidyl peptidase-4; NC, Normal control (n=8); D-ISP, Diabetic–Isoproterenol control (n=9); TA, Terminalia arjuna (n=7)
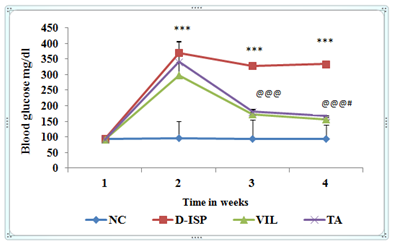
Figure 2 Time course changes in blood glucose levels among various experimental groups.
Values are expressed as mean±SD.
*** P<0.001 NC VS D-ISP; @@@p<0.001 D-ISP VS VIL,TA, #p<0.05 TA VS VIL.
NC, Normal control (n=8); D-ISP, Diabetic-isoproterenol control (n=9); VIL, Vildagliptin (n=8); TA, Terminalia arjuna (n=7)
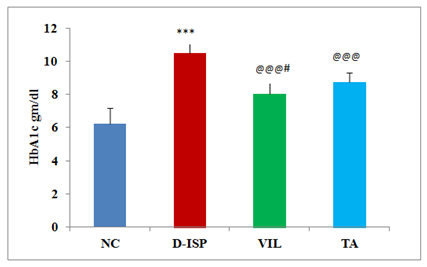
Figure 3 Glycosylated hemoglobin among various experimental groups.
*** P<0.001 NC VS D-ISP; @@@p<0.001 D-ISP VS VIL,TA, #p<0.05 TA VS VIL.
NC, Normal control (n=8); D-ISP, Diabetic-isoproterenol control (n=9); VIL, Vildagliptin (n=8); TA, Terminalia arjuna (n=7)
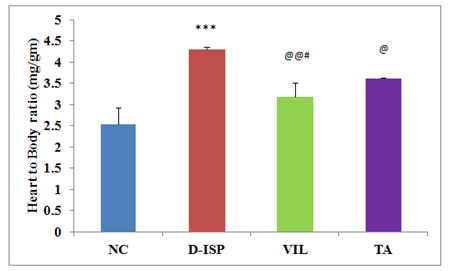
Figure 4 Heart to body weight ratio among various experimental groups.
Values are expressed as mean±SD.
*** P<0.001 NC VS D-ISP; @@@p<0.001 D-ISP VS VIL,TA, #p<0.05 TA VS VIL.
NC, Normal control (n=8); D-ISP, Diabetic-isoproterenol control (n=9); VIL, Vildagliptin (n=8); TA, Terminalia arjuna (n=7)
Histopathological section of myocardium
Photomicrograph of NC group rat heart revealed the non-infracted architecture of the myocardium (Plate A). In contrast, D-ISP group rat heart showed fatty infiltration in myocardial cells, hemorrhage, and marked edema, separation of myofibers, congested blood vessels and inflammation as compared to the NC group (Plate B). In the VIL treatment group rats, occasional focal myofibers loss, less inflammation, necrosis and edema was observed (Plate C). In the TA treatment group rats, occasional focal myofibers loss, inflammation, necrosis and edema was observed (Plate D) (Figure 7).
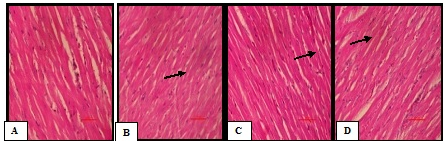
Figure 7 (A-D) Histopathology of the Heart: Representative photographs demonstrating myocardial tissue sections stained with H&E among various experimental groups.
A: Normal control– Normal architecture of the myocardium;
B: Diabetic –Isoproterenol control-Marked edema, confluent areas of necrosis and separation of myofibers, congested blood vessels, inflammation and haemorrhage;
C: Vildagliptin – Less inflammation, edema was observed;
D: Terminalia arjuna –Occasional focal myofiber loss, less inflammation, necrosis and edema was observed. Arrows indicate separation of myofibers congested blood vessels inflammation.
Scale bar = 100 𝜇m.
Histopathological section of pancreas
Photomicrograph of pancreatic sections of NC rats showed an organized pattern and normal architecture of islets of langerhans and the beta cells (Plate A). In contrast, the pancreas of D-ISP group rat showed severe degenerative changes in the pancreatic islets, damaged islets of langerhans, reduced beta cell mass and the atrophy of beta cells with the loss of few nucleus and cytoplasm and inflammatory infiltration (Plate B). Treatment group rats pancreas showed improved beta cell mass, less fibrosis, less inflammatory infiltration and hemorrhage as compared to D-ISP group (Plate C,D) (Figure 8), (Table 2).
SNO |
Variables/Groups |
Pancreatic Marker Lipase(U/L) |
Liver Marker SGPT(U/L) |
Renal Marker Creatinine (mg/dl) |
1 |
NC |
30.36+1.15 |
61.25+8.65 |
0.32+0.07 |
2 |
D-ISP |
42.46+4.11*** |
84.54+5.57*** |
0.61+0.05*** |
4 |
VIL |
38.53+3.62 |
74.36+8.68@@ |
0.48+0.07@ |
5 |
TA |
33.68+0.9@@@# |
68.35+3.62@@ |
0.39+0.05@@# |
Table 2 Safety parameters among various experimental groups
Values are expressed as mean±SD.
***p<0.001 NC VS D-ISP;
@@@ p<0.001;
@@ p<0.01,
@ p<0.05D-ISP VS VIL,TA;
## p< 0.01,#<0.05 VIL VS TA.
CPK-MB, Creatinine phosphokinase-MB; hs-CRP, High sensitive reactive protein; DPP-4, Dipeptidyl peptidase-4; NC, Normal control (n=8); D-ISP, Diabetic–Isoproterenol control (n=9); TA, Terminalia arjuna (n=7)
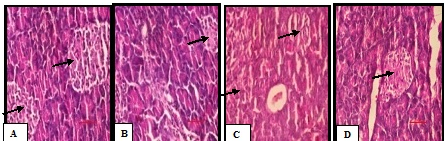
Figure 8 (A-D) Representative photographs demonstrating pancreatic tissue sections stained with H&E among various experimental groups.
A: Normal control–Organized pattern and normal architecture of islets of langerhans and the beta cells mass;
B: Diabetic –Isoproterenol control-Damaged islets of langerhans, atrophy of beta cells and reduced beta cell mass;
C:Vildagliptin - Improved beta cell mass, less fibrosis, less inflammatory infiltration and no hemorrhage;
D: Terminalia arjuna – Improved beta cell mass, less inflammatory infiltration and no hemorrhage as compared to D-ISP group. Arrows indicate the beta cells mass.
Scale bar = 100 𝜇m.
Safety of natural DPP-4 inhibitors: terminalia arjuna therapy
As seen from the Table 2, it was found that the in D-ISP group a significant elevation in the levels of pancreatic lipase (U/L) (p<0.001), SGPT (U/L) (p<0.001) and Creatinine (mg/dl) (p<0.001) was observed at 5th week compared to NC group. The treatment groups did not adversely affect the pancreatic, liver and kidney function in myocardial infarction co-existing with diabetes rats, as evidenced by pancreatic, hepatic and renal biochemical markers of injury as well as histopathological studies.
Safety of natural DPP-4 inhibitors: terminalia arjuna therapy
As seen from the Table 2, it was found that the in D-ISP group a significant elevation in the levels of pancreatic lipase (U/L) (p<0.001), SGPT (U/L) (p<0.001) and Creatinine (mg/dl) (p<0.001) was observed at 5th week compared to NC group. The treatment groups did not adversely affect the pancreatic, liver and kidney function in myocardial infarction co-existing with diabetes rats, as evidenced by pancreatic, hepatic and renal biochemical markers of injury as well as histopathological studies.
Histopathological section of Liver
Photomicrograph of the liver of the NC group (Plate A) rats, showed normal architecture of central vein, peripheral vein and hepatocytes. In contrast, the liver cells of the D-ISP group (Plate B) showed degeneration, scattered necrotic cells, congestion in the central vein as compared to NC group. However VIL treatment (Plate C) decreased the granular degeneration as compared to D-ISP rats. In TA treated group (Plate D) mild granular degeneration, inflammatory infiltration, edema, necrosis, hepatocytes degeneration which was less compared to standard drug group was observed (Figure 9).
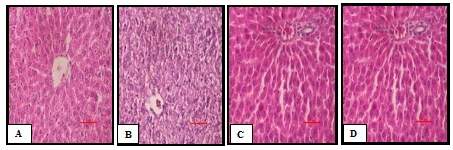
Figure 9 (A-D) Representative photographs demonstrating histopathological finding of liver tissue sections stained with H&E among various experimental groups.
A: Normal control–Normal architecture of central vein, peripheral vein & hepatocytes;
B: Diabetic–Isoproterenol control-scattered necrotic cells, congestion in the central vein;
C :Vildaglipti–Less granular degeneration, inflammation and necrosis;
D:Terminalia arjuna –Very Less granular degeneration, inflammation, edema, necrosis;
Scale bar = 100 𝜇m.
Histopathological section of Kidney
Photomicrograph of NC group kidney (Plate A) showed normal structure of the kidney, absence of congestion of glomerular blood vessels, tubular necrosis and inflammation. In contrast, histological assessment of the D-ISP group (Plate B) demonstrated marked congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration. In VIL treated group rats kidney showed less congestion of glomerular blood vessels, inflammation and focal area (Plate C). In TA group rats, renal tissue section showed less tubular necrosis and inflammation (Plate D) (Figure 10).
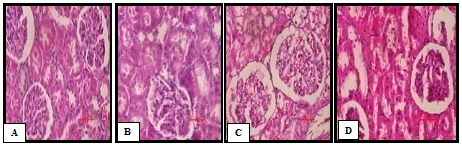
Figure 10 (A-D) Representative photographs demonstrating histopathological finding of kidney tissue sections stained with H&E among various experimental groups.
A: Normal control–Normal structure of the kidney;
B: Diabetic–Isoproterenol control-Marked congestion of glomerular blood vessels, tubular necrosis, inflammation and cloudy degeneration;
C: Vildagliptin–Mild tubular necrosis, inflammation and edema;
D: Terminalia arjuna–less tubular necrosis and inflammation;
Scale bar = 100 𝜇m.
Terminalia arjuna belongs to family Combretaceae, commonly known as Arjuna has traditionally been used in the Indian system of medicine for several medicinal purposes; cardiotonic, antidiabetic, antidysenteric, antipyretic and astringent.10 There is scientific evidence for the beneficial effects of Terminalia arjuna on diabetes mellitus and cardiovascular diseases. Previous study reported from the laboratory that Terminalia arjuna, significantly inhibits DPP-4 enzyme.22 In the present study, for the first time the cardioprotective efficacy and safety of this natural DPP-4 inhibitor Terminalia arjuna was evaluated in the setting of diabetes using an experimental model of myocardial infarction co-existing with diabetes. Terminalia arjuna therapy demonstrated significant cardioprotective effects in experimental model of myocardial infarction co-existing with diabetes. Myocardial salvaging effects of Terminalia arjuna treatment protocols may be attributed to several biochemical (Antidiabetic {Blood glucose, HbA1c}, cardiac {CPK-MB}, inflammatory marker {hs-CRP}, DPP-4 pathway {Serum DPP-4}, safety markers {Pancreatic function [lipase], liver function [SGPT], kidney function [Creatinine]}. Histopathological and biochemical markers of injury confirmed the safety of Terminalia arjuna on the pancreatic, hepatic and renal functions. The present study confirmed the hypoglycaemic effects of hydroalcoholic extracts of Terminalia arjuna in diabetic rats challenged with Isoproterenol. A significant decrease in the blood glucose and HbA1c levels was observed in the VIL and TA treated groups as compared to D-ISP control group. Observed antidiabetic activity of Terminalia arjuna was supported by M. Biswas et al.,23 Siddique et al.,11 Ragavan B et al.12 Terminalia arjuna has been reported to increase peripheral glucose utilization and enhanced insulin action. Terminalia arjuna contains the active principles like glycosides, alkaloids, terpenoids, flavonoids etc., have antioxidant activity. Antioxidant treatment has beneficial effects on preservation of β‑cell function in diabetes and also Flavonoids are known to regenerate the damaged β‑cell in the alloxan induced diabetic rats.24,25 It may have exerted its beneficial effect by its antioxidant activity (Figure 2) (Figure 3). The present study for the first time reported the cardioprotective efficacy of natural DPP-4 inhibitor: Terminalia arjuna in the presence of diabetes based on various biochemical and histopathological studies. The heart to body weight ratio, is considered as an index of cardiac hypertrophy. Boluyt et al.26 demonstrated that continuous infusion of ISP in rats elicits typical cardiac gene expression and hypertrophy due to pressure overload.26 Similar to ISP administration, STZ also results in an increase in fibrous tissue and collagen, causing an increase in the heart to body weight ratio in diabetes. TA and VIL treatment resulted in a significant reduction in the heart to body weight ratio as compared to D-ISP rats. The protective effect of Terminalia arjuna on cardiac hypertrophy was supported by Santosh Kumar et al.27 In addition treatment with TA and VIL significantly restored elevated serum CPK-MB levels as compared to D-ISP group. However, VIL therapy demonstrated superior cardioprotective efficacy as compared to TA. This cardio protective finding of Terminalia arjuna was in agreement with an earlier studies reported by Shukla et al.28 and Adila Parveen et al.29 in ISP induced cardiotoxicity. (Figure 4-6), (Table 1) The protective effects of Terminalia arjuna on myocardial injury demonstrated by biochemical parameters was confirmed by histopathological assessment. Terminalia arjuna treatment resulted in structural improvement in myocardium of experimental rats. Reduced edema, inflammation and necrosis was observed as compared to D-ISP group. Such an observation was in accordance with earlier reports by Sukla et al.28 (Figure 7). In addition to cardioprotection, TA and VIL treated rats showed significant reduction in serum DPP-4 levels as compared to D-ISP group; one of the mechanisms that explain their anti-hyperglycemic activity. Strikingly, the decrease in serum DPP-4 levels positively correlated with the cardiac injury marker CPK-MB (Figure 6). Thus, DPP-4 inhibition has beneficial effects on the heart. Interestingly, the DPP-4 inhibitory activity of Terminalia arjuna has been reported for the first time in the present study. DPP-4 has 3 major functions; adenosine deaminase binding, peptidase activity, and extracellular matrix binding, all of which potentially influence the activity of the immune and/or endocrine systems.30 Although DPP-4 cleaves and inactivates several cardioactive peptides, including neuropeptide Y, BNP, SDF-1 and GLP-1. Our observations demonstrating that inhibition of DPP-4 is associated with restored elevated serum CPK-MB, suggests that DPP-4 modifies cardiovascular outcomes.
The present study also evaluated the safety of standard drugs and test drugs on the vital organs: pancreas, liver and kidney. The markers of pancreatic function (pancreatic lipase), liver function (SGPT), kidney function (Creatinine) were assessed in addition to histopathological evaluation of the degree of injury. Increased pancreatic lipase levels as seen in D-ISP rats showed presence of pancreatic tissue damage. This was attenuated by various treatment protocols, there by restoring the architecture of the pancreas. This is the first report of the effect of test drugs Terminalia arjuna on the pancreatic function in the experimental model of myocardial infarction in setting of diabetes. Terminalia arjuna therapy did not adversely affect the hepatic and kidney function as evidenced by liver and renal function biochemical markers as well as histopathological studies. Earlier report by Chanan kumar et al.31 supported that Terminalia arjuna does not adversely affect liver and kidney function. Evidence from several studies have suggested that DPP-4 inhibitors improve cardiac function in both animal and clinical studies.32–34 In order to delineate if cardioprotective effects of Terminalia arjuna is attributed to DPP-4 inhibition, Serum DPP-4 levels were estimated in the various experimental groups. Terminalia arjuna and Vildagliptin treatment, restored the elevated DPP-4 levels observed in the D-ISP rats. Vildagliptin treatment showed superior reduction in serum DPP-4 levels as compared to Terminalia arjuna. It was also found that the cardioprotection (as indicated by cardiac marker CPK-MB levels) demonstrated by Terminalia arjuna and Vildagliptin was found to positively correlate with serum DPP-4 levels indicating that modulation the DPP-4 pathway contributes to their cardioprotective efficacy. Thus, the present study demonstrated the DPP-4 inhibition contributes to the myocardial salvaging effects of Terminalia arjuna in the setting of diabetes.
Terminalia arjuna demonstrated beneficial effects in experimental model of myocardial infarction co-existing with diabetes.
None
None.

©2018 Borde, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.