Journal of
eISSN: 2374-6947


Research Article Volume 8 Issue 1
1Department of Internal Medicine/Endocrinology, University of New Mexico Health Sciences Center, USA
2Medical Associates of Northern New Mexico, USA
3Vancouver Clinic, USA
Correspondence: Mark R Burge, M.D, Regents’ and Distinguished Professor of Medicine, University of New Mexico Health Sciences Center, Department of Medicine/Endo–5ACC, MSC10-5550, 1 University of New Mexico, Albuquerque, NM, USA
Received: December 10, 2020 | Published: February 25, 2021
Citation: Fallahi I, Garimella M, Mitchell S, et al. Effects of insulin detemir versus insulin glargine on food intake and satiety factors in type 1 diabetes. J Diabetes Metab Disord Control. 2021;8(1):31‒36. DOI: 10.15406/jdmdc.2021.08.00218
Background: Insulin detemir is long-acting insulin analog that is weight-neutral compared with other long-acting insulins in patients with type 1 diabetes. One mechanism for this may be an effect of insulin detemir to enhance satiety. We hypothesized that type 1 diabetes patients on insulin detemir will eat fewer calories when presented with a standardized buffet meal following a 24-hour fast as compared to those on insulin glargine.
Methods: Ten subjects with C-peptide negative type 1 diabetes participated in a randomized, double-blind crossover study in which they received equivalent doses of either insulin detemir or insulin glargine twice daily for at least 3 weeks. They were subsequently admitted to the UNM Clinical Research Unit for a 24-hour fast, after which they were allowed to eat to satiety from a standardized buffet. Caloric consumption, hunger score and body compositions were measured. Leptin, Ghrelin and Peptide YY were assessed at baseline, after 24-hour fast, and after ingestion of the meal.
Results: Subjects were aged 35±11 years, had diabetes for 18±11 years, had A1c levels of 8±1% and BMI of 30±8 kg/m2. Short acting insulin doses were higher for subjects receiving insulin detemir versus insulin glargine (p<0.001). Hunger scores, total energy ingested following the 24-hour fast, and Resting Energy Expenditure did not significant differ between the two study conditions.
Conclusion: The weight-neutrality of insulin detemir in type 1 diabetes is not attributable to reduced caloric intake following a fast, or to serum satiety factors.
Keywords: type 1 diabetes, insulin treatment, hunger and satiety
T1D, type 1 diabetes mellitus; NPH, neutral protamine hagedorn; GCRC, general clinical research center; CTSC, clinical and translational science center; POMC, proopiomelanocortin; NPY, neuropeptide Y
The underlying pathophysiology of type 1 diabetes mellitus (T1D) results in absolute insulinopenia1,2 and a tendency towards ketosis.3 As such, insulin analogues (including the animal insulins) have been the mainstay of therapy in T1D for most of the past 90 years. Previous studies have demonstrated that intensified insulin therapy causes weight gain, increased insulin doses, and an increased frequency of hypoglycemia.4 As such adherence to prescribed insulin regimens in type 1 diabetes may be compromised in attempt to avoid weight gain. A longitudinal study in the United Kingdom among 65 patients with T1D showed that up to 30% of females acknowledged under-dosing their insulin in effort to control their weight.5 Accordingly, a number of studies have been performed comparing different long acting insulins with respect to their effects on glycemic control, hypoglycemia, and body weight. A growing body of evidence indicates that the long acting basal insulin analogue insulin determir has weight sparing effects.6 Most of the studies performed to date have compared insulin detemir with isophane Neutral Protamine Hagedorn (NPH) insulin, with the results showing that insulin detemir is associated with less weight gain and a lower risk of hypoglycemia in both type 1 and type 2 diabetes.7,8
A study by Raskin et al.,9 compared insulin glargine with NPH insulin over 16 weeks in patients with T1D and demonstrated decreased fasting blood glucose and decreased weight gain in the insulin glargine group as compared to NPH insulin.9 Nevertheless, the effects of insulin detemir and insulin glargine on body mass, body composition, energy expenditure, and hunger or satiety factors have not been previously evaluated in a head-to-head study. As such, we designed a study to compare the effects of insulin detemir and insulin glargine on caloric homeostasis in type 1 diabetes. Based upon previous studies comparing it to NPH, we hypothesized that type 1 diabetes patients would consume fewer calories when presented with a standardized buffet meal following a 24-hour fast when they were using insulin detemir as compared to when they were using insulin glargine.7
Study subjects
Ten subjects with C-peptide negative T1D participated in a randomized, double-blind crossover study. Inclusion criteria included ages 18-60 years, treatment with insulin therapy for at least 2 years, A1c value less than or equal to 11%, and c-peptide values less than 1.0 pmol/ml 90 minutes after stimulation. Exclusion criteria included advanced complications of diabetes (e.g.-nephropathy, retinopathy, neuropathy, or coronary artery disease); severe medical illness or medical conditions (e.g.-congestive heart failure, angina, liver failure, or renal failure); pregnancy; alcohol or drug abuse or dependence within three months of study entry; and less than 50% agreement on a 50-item food questionnaire detailing the “buffet style” food array to be used in the study meal.
Screening
Subjects underwent a screening visit at the outpatient clinic of the UNM General Clinical Research Center (GCRC). This visit included a complete physical examination, a review of the subject’s chart and medical history, and laboratory testing. Blood was obtained for c-peptide 90 minutes after oral administration of Boost Plus® (6 mg/kg up to 360 ml over five minutes, Nestle HealthCare Nutrition, Inc., Bridgewater, NJ), as well as a basic metabolic panel, A1c, urine pregnancy test, and a 50-item food questionnaire to assess food preferences. The 50-item food questionnaire was used to ensure that subjects consumed the items that were to be presented in the study meal.
Study protocol
After an initial screening visit to determine eligibility and to obtain informed consent, study subjects were randomized into two groups. A random-number generator was used to determine the order in which subjects received the study insulins. Even numbers received insulin glargine first while odd numbers started with insulin detemir, and then subjects crossed over to the alternative insulin analogue. Subject blinding was achieved by transferring both study insulin types to identical appearing vials by the UNM HSC Investigational Pharmacy. Subjects were instructed to inject the study insulin subcutaneously twice daily for 3 weeks at a prescribed dose. If patients were not using either insulin detemir or insulin glargine as part of their usual insulin regimen, they were transitioned to equivalent doses of the study insulin. Subjects were instructed to inject the study insulin at 0800 and 2000 hours each day, and they remained on their current dose of rapid acting mealtime insulin. Subjects recorded capillary blood glucose readings four times daily (before meals and at bedtime) and were asked to contact the GCRC outpatient nursing staff during the first three days of therapy to relay their CBG values. Insulin doses were titrated to obtain fasting glucose values of below 180 mg/dl, with a goal glucose value below 150 mg/dl. After three weeks of each assigned therapy, subjects were admitted to the GCRC inpatient unit at 1300, and intravenous access was established. At 1500, subjects were administered their usual dose of subcutaneous rapid acting mealtime insulin and were given a standardized, 8 kcal/kg ADA meal, consisting of 50% carbohydrates, 20% protein, and 30% fats.
After the standardized meal, subjects fasted with the exception of free access to non-caloric, non-caffeinated beverages. At 2000, subjects were administered their usual dose of study insulin (insulin detemir or insulin glargine) subcutaneously. Between 0300 and 0700 hours, subjects received intravenous regular insulin by infusion only if needed to maintain blood glucose between 90-120 mg/dl. For this infusion, intravenous regular insulin was mixed in 0.45 mol/L saline to a concentration of 0.1 U/ml. Capillary blood glucose values were assessed at baseline, at 15 minutes and 30 minutes after initiating the IV insulin infusion, and then every 30 minutes thereafter for a total of four hours. At 0700, intravenous insulin was discontinued (if applicable), and subjects underwent measurement of Resting Energy Expenditure via Indirect Calorimetry, as well as Bioelectrical Impedance Analysis to ascertain body composition. At 0800, subjects again received their usual dose of study insulin subcutaneously. At 1500, after 24 hours of fasting, subjects were presented with a “buffet style” study meal prepared by the GCRC dietician, who consisted of a 10,000 kcal food array as previously described, and subjects were allowed to eat until satiety.10 Following the meal and final data collection, subjects were provided their usual dose of subcutaneous rapid acting mealtime insulin and were monitored for at least 30 minutes. At 1600, the study ended and subjects were discharged home.
Serum leptin, ghrelin and PYY concentrations were collected on admission, at 1450 (just prior to study meal presentation), and at 1600 (60 minutes after the study meal). Serum glucose and insulin values were measured every 2 hours during hospitalization. Subjects completed Three Factor Food Questionnaires and Visual Analogue Scales for satiety at three time points during the study: immediately upon hospital admission, and then again after 12 and 24 hours of fasting.11,12 Subjects also completed a 24-hour dietary recall during the hospitalization to characterize their diet at home. Subjects then crossed-over to the alternative study insulin for 3 weeks, upon completion of which they were readmitted to the UNM GCRC to repeat the above protocol. At the conclusion of the second inpatient admission, subjects returned to their pre-study insulin regimens. All subjects rendered informed consent as approved by UNM Human Research Review Committee, and the study was registered on clinicaltrials.gov (NCT #00659165).
Sample analysis
Whole blood capillary blood glucose concentrations were determined with a bedside One Touch® glucometer (Lifescan, Milpitas, CA). Plasma glucose was determined by the UNM Clinical and Translational Science Center (CTSC) Translational Laboratory using the ACE Glucose Reagent (Alfa Wassermann, Caldwell, NJ). Free insulin concentrations were determined using an Ultra-Sensitive Human Insulin radioimmunoassay (RIA, Linco, St. Charles, Missouri) after treatment of the samples with polyethylene glycol to precipitate any circulating anti-insulin antibodies. This assay employs the double antibody/PEG technique to achieve a sensitivity of 0.2 microU/mL when using a 100 microliter sample. C-peptide concentrations were determined using radioimmunoassay (INCSTAR, Steelwater, MN). A1c was assayed using a spectrophotometetric method (Bayer DCA 2000 Analyzer, Kernersville, NC). Serum ghrelin concentrations were determined at the UNM CTSC Translational Laboratory by RIA using the LINCO kit (St. Charles, MO). Leptin and Peptide-YY concentrations were determined by the University of Colorado Clinical and Translational Science Center Translational Laboratory. Leptin levels were determined using a commercial ELISA assay from Millipore Corp. (Burlington, MA), while PYY levels were determined using an RIA from Millipore Corp. (Burlington, MA).
For results presented, Mean ± Standard Deviation are provided in the text, and Mean ± Standard Error of the Mean are depicted in the figures.
Statistical analysis
The primary outcome variable of the study was calories consumed while eating to satiety after a 24-hour fast. The total caloric intake of subjects with T1D was estimated to be 2500 calories per day, equaling approximately 833 calories per meal. Sample size determination revealed 82% power to detect a 15% difference in calories consumed when eating to satiety between the insulin detemir and insulin glargine treatments with alpha equal to 0.05 using a paired two-sample t-test to analyze the differences in calories consumed. Secondary outcome variables included the serum hunger and satiety factors leptin, ghrelin, and PYY to provide characterization of the modulation of energy intake by the insulin analogues, with comparison of these factors between the different study conditions using paired t-tests. Plasma free insulin and glucose concentrations were measured to assess the rate of systemic insulin absorption. Three Factor Food Questionnaires and Visual Analogue Scales of satiety, food diaries and 24-hour dietary recall data were used to characterize potential differences in appetite modulation between the insulins. Binary responses were compared using McNemar’s test for repeated binary variables. Home capillary blood glucose monitoring data were used to assess compliance to therapy and to ensure similar glycemic control in both arms of the protocol. Body composition as determined by Bioelectrical Impedance Analysis was used to determine if insulin type altered body fat content, and indirect calorimetry was used to determine potential differences in Resting Energy Expenditure.
Patient characteristics
The ten subjects (7 females and 3 males) were aged 35±11 years, had T1D for 18±11 years, had baseline A1c levels of 8±1%, and a mean baseline BMI of 30±8 kg/m2. All enrolled subjects were C-peptide negative (less than 1 pmol/ml) during hyperglycemia. Figure 1 shows the CONSORT diagram for this study.
Primary and secondary outcomes
As shown in Figure 2, home glucose values did not differ during treatment between the insulins during the study (187±254 for detemir vs.172±269 mg/dl for glargine; p=0.21), but short acting insulin doses were higher for insulin detemir as compared to insulin glargine (15±10 vs. 13±8 units/day; p<0.001). Hunger scores (68±25 vs. 73±24; p=0.58) and total energy ingested following the 24-hour fast (1418±636 vs. 1357±576 kcal; p=0.63) did not differ between insulin detemir and insulin glargine, respectively. Resting Energy Expenditure also did not differ (1405±398 vs. 1457±536 kcal/d; p=0.63). BMI did not differ between insulin detemir and insulin glargine at the end of each three week study condition (29.9±8.9 vs. 30.7±8.7, p=0.16). Percent body fat was 34.4±12.2% in insulin detemir group as compared to 35.4±11.3% in insulin glargine group, with no statistically significant difference observed (p=0.2, Figure 3). Mean daily total energy intake prior to admission for the overnight study was 1419±637 kcal during insulin detemir therapy and 1357±577 kcal during insulin glargine therapy (p=0.49). Nutritional intake also did not differ by type according to dietary recall, with carbohydrate intake (171±82 grams for insulin detemir vs. 160±58 grams for insulin glargine, p=0.48), fat intake (59±31 grams for insulin detemir vs. 56±34 grams for insulin glargine, p=0.59), or protein intake (60±24 grams for insulin detemir vs. 63±25 grams for insulin glargine, p=0.39) all resulting in statistically insignificant comparisons. As shown in Figure 4, there were no differences in ghrelin, leptin, or PYY concentrations between the study conditions.
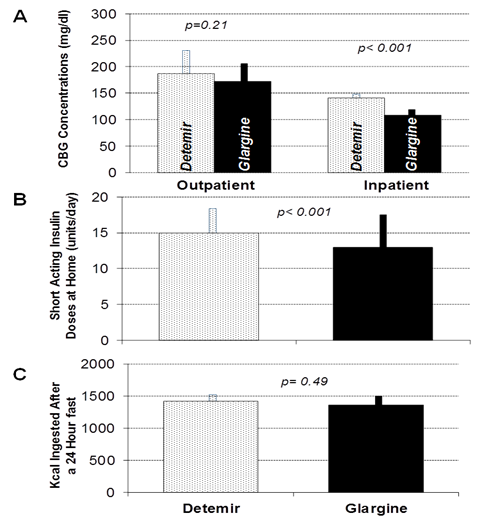
Figure 3 (A) Mean home capillary blood glucose (CBG) concentration among 10 patients who sequentially received insulin glargine and insulin detemir for three weeks in random order (left), and mean capillary blood glucose concentrations during inpatient hospitalizations for 24-hour fasting studies with each long acting insulin (right). (B) Mean doses of premeal rapid-acting insulin analogues used at home during each study condition. (C) Mean energy ingested from a 10,000 kcal food array following the 24-hour fasting studies for insulin detemir (stippled bars) and insulin glargine (solid black bars). Error bars depict standard error of the mean.
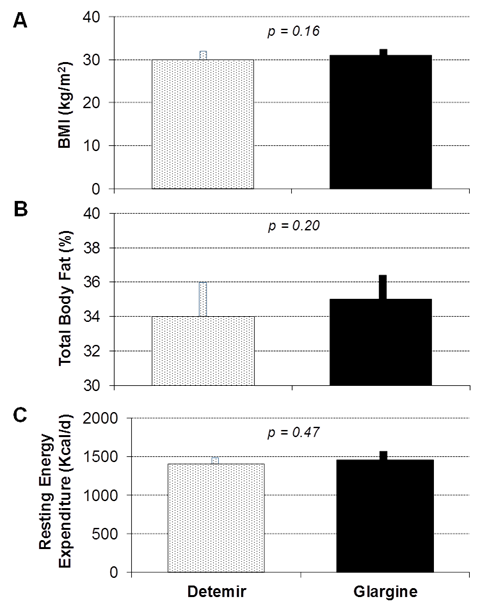
Figure 4 Mean Body Mass Index (A), Total Body Fat as determined by Bioelectrical Impedance (B), and Resting Energy Expenditure during a 24-hour fast (C) following three weeks of therapy with insulin detemir (stippled bars) and insulin glargine (solid black bars). Error bars depict standard error of the mean.
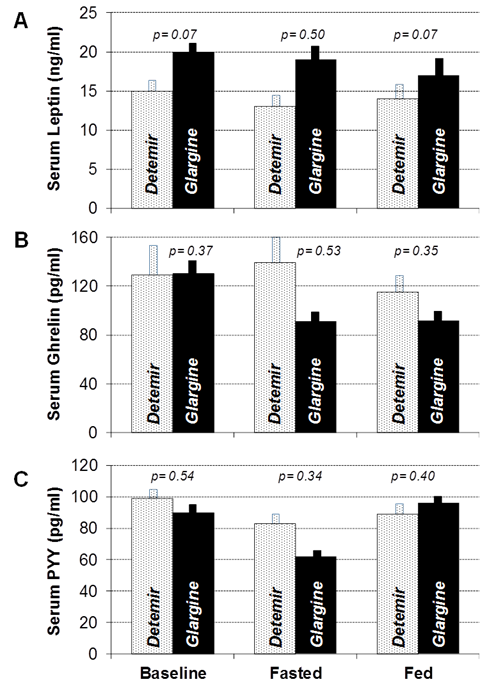
Figure 5 Mean Serum Leptin (A), Ghrelin (B), and Peptide-YY (PYY, panel C) concentrations collected before and after 24-hour fasting studies and after meal ingestion following three weeks of therapy with insulin detemir (stippled bars) and insulin glargine (solid black bars). Error bars depict standard error of the mean.
This six week randomized, double-blind clinical trial describes the first head-to-head comparison of calorie consumption and energy expenditure between the two currently most popular long-acting insulin analogues for patients with type 1 diabetes, insulin detemir and insulin glargine. These results suggest that there are no significant differences between the parameters we assessed, so the mechanism of the purported weight neutrality of insulin detemir was not identified in this study. Nevertheless, existing evidence has indicated that insulin detemir has a weight sparing effect relative to older insulin therapies, such as NPH.6 Numerous theories have been proposed for the putative weight-neutral effects of insulin detemir, including a reduction in hypoglycemic episodes resulting in fewer calories ingested, less fluid retention, stimulation of the central nervous system for improved satiety, and preferential inhibition of hepatic glucose production.13 A study by Hallschmid et al.,14 compared insulin detemir to Regular Human Insulin with respect to food intake and showed that while both insulins had comparable effects, insulin detemir exerted stronger acute effects on brain function as measured by electroencephalogram and triggered a relative decrease in food consumption as compared to human insulin.14 The process through which insulin detemir mediates this effect on the CNS is unclear, but one of the postulated theories is that binding of detemir to albumin enhances active transport across the blood brain barrier which consequently raises the insulin concentration of brain tissue, correlating with increased blood flow to appetite-related regions of the brain such as the insular cortex and the thalamus.6,15 In addition, a study done by Van Golan et. al, showed that Insulin detemir decreased body weight by 0.8 kg and NPH insulin increased weight by 0.5 kg (p=0.02). There was significantly lower brain activation in bilateral insula, where it is assumed to be involved in food choices, in response to visual food stimuli during insulin detemir therapy compared to NPH.16
Several other centrally acting neuroendocrine peptides have been proposed to influence hunger and satiety, and it is conceivable that insulin detemir may act through these peptides to modulate appetite. For instance, one neuronal circuit inhibits food intake via the expression of proopiomelanocortin (POMC), and another stimulates food intake through expression of Neuropeptide Y (NPY).15–18 Leptin is a peptide hormone secreted by adipose tissue that is transported across the blood brain barrier to activate POMC neurons, thereby inhibiting food intake.17,18 Conversely, ghrelin is released by the oxyntic cells of the stomach, small-, and large intestine that serves as a hunger signal during fasting by stimulating NPY neurons centrally, thereby increasing food intake.17,18 Peptide tyrosine-tyrosine (Peptide YY, PYY) is released mainly from the gastrointestinal tract in response to feeding and acts as an agonist of the Y2 receptor in the hypothalamus. When activated, the Y2 receptor inhibits the release of NPY and suppresses appetite.19 In our study, leptin levels were appropriately decreased from baseline after fasting and increased after feeding under both the insulin glargine and insulin detemir study conditions. Leptin concentrations also did not differ between study conditions at baseline, fasted or fed states. Similarly, PYY levels were appropriately decreased during the fasting state and increased after feeding with both insulin analogues and the groups did not significantly differ with respect to PYY concentrations. Finally, ghrelin concentrations were appropriately increased after fasting in the insulin detemir group but decreased in the insulin glargine group relative to baseline. In the insulin detemir group, ghrelin appropriately decreased after eating but remained relatively unchanged in the insulin glargine group. Even though, these differences in concentration did not achieve statistical significance with between-group comparison, it is consistent with a previous study done by Makino et al.,20 showing that glargine treatment significantly suppressed acyl ghrelin levels whereas detemir therapy did not. This could be potentially the reason why the patients treated with glargine did not have increased BMI compared to patients receiving detemir given the fact that ghrelin induces a positive energy balance, an increase in adiposity, as well as an increase in caloric storage.20,21
As such, the results of our study indicate that insulin detemir does not have central nervous system effects on appetite or satiety in humans as compared to insulin glargine in type 1 diabetes. This finding is in contradistinction to the findings in rats, where whole body energy expenditure is significantly increased with insulin detemir after 72 hours as compared with regular human insulin.22 In the same study, there was reduced food intake in insulin detemir group compared to regular human insulin.22 Previous studies have shown an effect of insulin detemir to reduce food intake as compared to NPH insulin, but NPH is also known to cause more frequent hypoglycemia compared to insulin glargine or detemir.2,23–25 Reduced nocturnal hypoglycemia may reduce the need for additional caloric intake by patients taking detemir as compared to patients taking NPH.8,26 However, data are mixed with regards to the effects of insulin detemir and insulin glargine on body weight. For example, a study done by Pieber et al.,27 compared glycemic control and the risk of hypoglycemia with twice daily insulin detemir to once daily insulin glargine in subjects with type 1 diabetes but did not demonstrate any significant differences between these two insulin analogues.27 This could be due to insulin glargine’s molecular structure and pharmacokinetic properties, where subcutaneous micro-precipitates are formed that dissolve slowly, thus prolonging its duration of action upon injection into neutral pH subcutaneous tissues reducing the risk of hypoglycemia and its attendant food ingestion.3
On the contrary, a study by Rosenstock et al.,28 described a 52 week multinational, randomized, open label, parallel group study in which there were modest reductions in weight gain among patients who received insulin detemir as compared to those who received insulin glargine in patients with type 2 diabetes.28 Further research needs to be done to provide more evidence regarding the mechanisms of differences in body composition following treatment with different long acting insulins, as well as their effects on hunger and satiety. Our study had a number of strength and weaknesses. Strength of this study was the fact that it comprised a prospective, double-blind, randomized, crossover design, comparing insulin glargine and insulin detemir head-to-head by assessing the clinical and physiologic indicators of hunger and satiety. The doses that were used for insulin glargine and detemir were equivalent in each group. Limitations of this study include the fact that the sample size was small and the study was of short duration. In addition, the study was performed at a single site, and most of the participants were females.
In conclusion, the short-term use of insulin detemir and insulin glargine resulted in comparable caloric ingestion following a 24-hour fast, glucose control, weight change, serologic indicators of hunger and satiety, and energy expenditure in this randomized, double-blind clinical trial. Further comparisons of insulin detemir and insulin glargine with a longer duration of therapy may be required to more fully elucidate differences in energy homeostasis between long acting insulin analogues among patients with type 1 diabetes, if such differences exist.
None.
The author declares that there is no conflict of interest.
None.

©2021 Fallahi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.