Journal of
eISSN: 2374-6947


Case Report Volume 2 Issue 5
1Department of Internal Medicine, Canakkale State Hospital, Turkey
2Department of Internal Medicine, Canakkale State Hospital, Turkey
3Department of Radio diagnosis, Canakkale State Hospital, Turkey
4Department of Pathology, Canakkale State Hospital, Turkey
Correspondence: Sema Ciftci Dogansen, Department of Internal Medicine, Canakkale State Hospital, 17100, Canakkale, Turkey, Tel +90286 217 10 98, Fax +90 286 213 69 01
Received: October 21, 2015 | Published: December 19, 2015
Citation: Dogansen SC, Soyler C, Sertcelik G, et al. Diabetic hyperosmolar nonketotic coma induced by central diabetes insipidus. J Diabetes Metab Disord Control. 2015;2(5):166-169. DOI: 10.15406/jdmdc.2015.02.00054
Introduction: Hyperosmolar hyperglisemic nonketotic coma (HONKC) is a rare complication of type 2 diabetes mellitus (DM). Polyuric dehydration caused by DI can lead to HONKC. One of the reasons of central diabetes insipidus (CDI) is metastatic malignancies. Most frequently breast and lung cancer and less frequently colorectal cancer can cause pituitary metastasis.
Case Presentation: A 59year old female, with a history of cervical lenf node metastasis and was being investigated for primary malignancy, admitted to emergency medicine service. She was diagnosed as HONKC with serious hypernatremia, hyperglycemia and hyperosmolarite. Although adequate hydration and normoglycemia was obtained, hypernatremia and polyuria persisted; therefore suspected diagnosis was diabetes insipidus (DI). In radiological investigation cerebral and pituitary metastatic lesions were seen, then with empirically desmopressin acetate treatment urinary output and polyuria recovered. In investigations to find primary malignancy; rectal wall was thickned and carcinoembryonic antigen levels were significantly increased. General condition was not suitable for colonoscopy and biopsy. Diagnosis was HONKC induced by CDI due to pituitary metastasis of probaple colorectal carcinoma.
Discussion: Polyuric dehydration caused by DI can lead to HONKC in type 2 diabetic patients. This rare togetherness should be considered in persistant hypernatremi and polyuria; although normoglycemia is provided. The most important sign of pituitary metastasis is DI. Because of this in metastatic malignencies with polyuria and polydipsia signs, pituitary metastasis should come to mind.
Keywords: diabetes mellitus, hyperosmolar hyperglisemic nonketotic coma, central diabetes insipidus, pituitary metastasis, polyuria, normoglycemia, thiazide diuretics, dehydration, enzyme inhibitor, potassium chloride, corticosteroids, hypernatremi
CEA, carcinoembryogenic antigen; CDI, central diabetes insipidus; COPD, chronic obstructive pulmonary disease; CRC, colorectal carcinoma; DI, diabetes insipidus; DM, diabetes mellitus; HONKC, hyperosmolar hyperglisemic nonketotic coma
Hyperosmolar hyperglisemic nonketotic coma (HONKC) is a rare but fatal complication of type 2 diabetes mellitus (DM).1 The most frequent precipitating factor is infections. Other precipitating factors are undiagnosed DM, drugs like corticosteroids and thiazide diuretics, diseases causing contregulatory hormon increase and poor compliance with diabetic medications.2 Furthermore, polyuric dehydration caused by diabetes insipidus (DI) can lead to HONKC. In literature there are cases both caused by nephrogenic and central DI (CDI).3-11
The causes of CDI can be diseases affecting hypothalamus and pituitary like inflammation, infections, trauma, neoplasm and autoimmune diseases; it can also be idiopathic.8 Pituitary metastasis cause DI mostly by posterior part involvement and rarely with infundibular involvement. Breast cancer is the most common tumor metastasizing to the pituitary gland, followed by lung, prostate, renal cell and gastrointestinal cancers have also been described.12 In particular, colorectal carcinoma (CRC) is a rare cause of metastasis to the pituitary gland.13 We present HONKC case induced by DI due to pituitary metastasis of probaple CRC.
A 59 year old female admitted to emergency medicine service of Canakkale State Hospital with confusion. She had comorbidities as chronic obstructive pulmonary disease (COPD) and hypertension. She had been treated with inhaler ß2 agonist and angiotensine converting enzyme inhibitor therapy. One month ago she admitted to internal medicine polyclinic with polyuria and polydipsia complaints continuing for a few months. Plasma biochemistry showed: impaired fasting glucose (glucose: 109 mg/dl, HbA1c: 6.1%), hypochromic microcytic anemia, erythrocyte sedimentation rate increased; serum electrolytes, renal and hepatic function test results were normal. Physical examination was normal except left cervical lenfadenopathy. Ultrasonography (USG) signs of lymph node was malign features; therefore excisional biopsy was performed and undifferantiated carcinoma metastasis was showed (Figure 1). In investigations to find primary malignancy mammography, breast USG, abdominal USG and thorax computed tomography (CT) were normal; only in abdominal CT rectal wall was thickened.
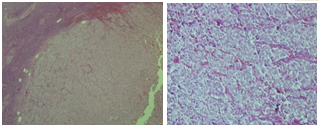
Fever, cough, dyspnea symptoms started on the last days, oral intake was disturbed and she was taken to emergency medicine with confusion. On physical examination she was uncounciouss, turgor tonus decreased, she had conjunctival paleness, no icteria, diffuse coarse rhonchus, expirium prolonged and was tachycardic. Her the calculation of body mass index (BMI) was 24 kg/m2 and the value was in the normal weight according to BMI classification.14 Blood pressure was 100/50 mmHg, heart rate was 112/min, body temperature was 38.5°C.
Plasma biochemistry showed; glucose: 676 mg/dl (range 75-100), blood urea nitrogen: 118 mg/dl (range: 10-40), creatinine: 2.1 mg/dl (range: 0.4-1.0), SGOT: 290 IU/L (range 10-42), SGPT: 129 IU/L (range 10-40), sodium: 172 mmol/L (range: 136-144), potassium: 3.9 mmol/L (range: 3.6-5.1), chloride: 130 mmol/L (range: 101-111); arterial blood gas analysis: pH: 7.38 (range: 7.35-7.45), pCO2: 43.2 mmHg (range: 35-45), pO2: 77 mmHg (range: 70-100), HCO3: 23.4 mmol/L (range: 21-26), base excess: 2.06 mmol/L; urinalysis showed no keton, blood, leucocytes, protein but glucose is positive, and density: 1005 g/ml; complete blood count: white blood cell: 15.8x10ł/mmł, hemoglobin: 9.8 g/dl (range: 11.7-15.5), hematocrit: 30.1% (range: 34.5-46.3), platelet: 150x10ł/mmł (range: 129-388), C-reactive protein: 9.8 mg/dL (range: 0-0.5), erythrocyte sedimentation rate: 107 mm/h (<20), HbA1c: 7% (range: 4-6). Calculated serum osmolarity was 402.5 mosm/lt (275-295) (Table 1). The diagnosis was diabetic HONKC precipated by pulmonary infection and the patient taken to intensive care unit.
Glucose |
676 mg/dl |
(75-100) |
Blood Urea Nitrogen |
118 mg/dl |
(10-40) |
Creatinine |
2.1 mg/dl |
(0.4-1.0) |
SGOT |
290 IU/L |
(10-42) |
SGPT |
129 IU/L |
(10-40) |
Sodium |
172 mmol/L |
(136-144) |
Potassium |
3.9 mmol/L |
(3.6-5.1) |
Chloride |
130 mmol/L |
(101-111) |
HbA1c |
7% |
(4-6) |
White Blood Cell |
15.8 x 10ł/mm |
(4.5-11.0) |
Hemoglobin |
9.8 g/dL |
(11.7-15.5) |
Hematocrit |
30.10% |
(34.5-46.3) |
Platelet |
150 x 10ł/mmł |
(129-388) |
Erythrocyte Sedimentation Rate |
107 mm/h |
(<20) |
pH |
7.38 |
(7.35-7.45) |
pCO2 |
43.2 mmHg |
(35-45) |
pO2 |
77 mmHg |
(70-100) |
Base Excess |
2.06 mmol/L |
(±2.0) |
C-reactive Protein |
9.8 mg/dl |
(0-0.5) |
Serum Osmolarity |
402.5 mosm/L |
(275-295) |
Urinalysis |
||
Density |
1005 g/ml |
(1010-1020) |
Keton |
Negative |
|
Glucose |
Positive |
|
Blood |
Negative |
|
Leucocytes |
Negative |
|
Protein |
Negative |
|
Table 1 The results of baseline laboratory tests
Corrected plasma sodium was 181 mmol/L and water deficit was approximately 7 liters. Initially intravenous (IV) fluid replacement started with hypotonic fluids, then 0.1 U/kg/h insulin infusion added and infusion rate regulated according to capillary blood glucose measurements. Potassium chloride was added to IV fluids. The patient was consulted to pulmonary disease department and antibiotic therapy started. Although blood glucose concentrations was between 100-200 mg/dl with insulin infusion therapy and enough water replacement; plasma Na concentration was always above 150 mmol/L and the patient remained polyuric, passing 6-8 litres of dilute urine daily (urine osmolality 175-288 mOsm/kg). Meanwhile her confusion recovered, she was conscious, renal and hepatic function test results were normal. Primarily HONKC triggered by infection was thought in the patient.
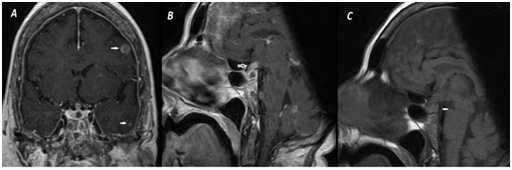
However, suspected diagnosis was DI because of sustained high dilute urinary output although obtained normoglycemia and persistent hypernatremia resistant to fluid replacement. Sella magnetic resonance imaging (MRI) was performed because of metastatic malignancy in history. Neurohypophysis high signal intensity loss secondary to stalk invasion in T1 weighted imaging (T1WI), stalk thickening and diffuse contrast distribution with contrasted T1WI, diffuse nodular contrasted focus due to intracerebral metastasis was showed (Figure 2). General condition of the patient was not suitable for water deprivation test. Therefore, desmopressin acetate treatment was started empirically; then urinary output improved and plasma Na concentrations was between 140-145 mg/dl.
Anterior pituitary hormon results was: TSH: 1.1 µIU/Ml (range: 0.34-5.6), FT4: 10.5 pmol/L (range: 7.9-14.4), FSH: 1.47 mIU/mL(range: 16-113), LH: 0.23 mIU/mL (range: 7.7-59), estradiol: 33.5 pg/mL (range: 10-40), cortisole: 11 µg/dL (range: 6.7-22), PRL: 41 ng/dl (5-25). There was no central hypothyroidism but had secondary hypogonadism, mild hyperprolactinemia due to pituitary stalk involvement and relative hypocortisolemia. Also because of cerebral metastasis; neurosurgery department added dexamethasone for antiedema effect.
In investigations to find primary malignancy; only rectal wall thickening was shown in abdominal CT. From tumor markers carcinoembryogenic antigen levels (CEA) were >978 ng/ml (range: 0-7). Colonoscopy was planned by gastroenterology department but not performed because of pulmonary dysfunction due to pneumonia and unstable general condition. Blood glucose concentrations was between 100-200 mg/dl, electrolyte imbalance was recovered but unfortunately the patient died because of respiratory failure.
The togetherness of DI and DM in the patient has been rarely reported in Wolfram syndrome (known as DIDMOAD) as a congenital genetic disorder in pediatric age group, which has the concurrent association of CDI with type 1 DM accompanied by optic atrophy, deafness, and infantilism.15 But togetherness of CDI and type 2 DM is rare.8 Polyuric dehydration caused by DI can occur in this togetherness. In literature there are cases both caused by central and nephrogenic DI.3-11
Lithium-induced nephrogenic DI is an important side effect in the patients treated with lithium. If there are the glucose intolerance in these cases with lithium-induced nephrogenic DI, they can develop HONKC. Such cases have been described in the literature.3,4 The togetherness of CDI and type 2 DM has been noted in less than 10 adult patients, some of whom had history of type 2 DM before developing CDI.10,11 An exception was a patient with Klinefelter’s syndrome who had CDI for more than 5 years before presenting with hyperosmolar coma due to type 2 DM.7
Furthermore, there are cases of the simultaneous development of CDI and type 2 DM reported similar to ours case in the literature.5,6,8,9 Vidyarthi et al.9 have identified a similar case to ours in recently. They have also started desmopressin as empirically due to persistent hypernatremi and sustained polyuria in their female patient with HONKC. Then, her sodium corrected to normal with 72 hours.
Most patients who develop HONKC are elderly; but cases on the caused by DI are mostly younger as in our patient.3 Also frequently there is a precipitating factor. These precipitating factors are mostly infections, poor compliance with diabetic medications or drugs like corticosteroids and thiazide diuretics.2 In cases caused by DI; hyperosmolarity can develop without additional factors. But our patient there were many risk factors like COPD, malignancy and DI existence. Even if DI and coma was treated, immunsupression due to metastatic malignancy caused the situation to be mortal in this patient.
The initiating event in hyperosmolar hyperglycemic state is glucosuric diuresis. Glucosuria impairs the concentrating capacity of the kidney, further exacerbating water loss. The loss of more water than sodium leads to hyperosmolarity. When adequate fluid replacement and maintained normoglycemia with treatment in HONKC, renal concentrating capacity improves and thus hypernatremia and diuresis recovers.16 In persistent hypernatremia and sustained polyuria, additional factors should be thought. Our patient suspected diagnosis was DI because of this reason. Because of unsuitable general condition water deprivation test not performed. Recovery with empirically desmopressin treatment and sella MRI findings support the DI diagnosis.
One of the reasons of CDI is pituitary metastasis. Posterior part of the pituiter gland is the most common site of metastasis, probably due to highly rich blood supply through the hypophyseal artery. Anterior part involvement is usually seen with posterior part involvement. Most of the pituitary metastasis are clinically silent. Clinical presentation of symptomatic pituitary metastasis is mostly as DI because it frequently affects posterior part. Other reported symptoms are ophtalmoplegia, headache, visual field defects and anterior pituitary disfunction.17 In a series with 190 symptomatic pituitary metastasis case DI was 45%, optic nerve involvement was 27.9%, anterior pituitary insufficiency was 23.6%.13
Our patient there is both DI due to posterior part and stalk involvement and partial anterior pituitary deficiency. Thyroid axis was normal but there was partial hypocortisolism and hypogonadism. Definitive diagnosis in metastatic disease is hystological evaluation. But most of the time this is not possible. In MRI findings high signal intensity loss of neurohypophysis and isointensity or hypointense mass is seen with T1 sequences; with T2 sequences high-intensity signal and homogeneous gadolinium involvement is seen.13 Our patient there was pituitary and cerebral metastasis compatible with these signs.
Breast cancer is the most common tumor metastasizing to the pituitary gland, followed by lung, prostate, renal cell and gastrointestinal cancers have also been described.12 In particular, CRC is a rare cause of metastasis to the pituitary gland. In two study CRC metastasis was shown to be between 2-2.4%.13,18 In this patient metastatic malignancy was the definitive diagnosis but colonoscopy and biopsy not performed because of unsuitable general condition. But rectal wall thickening in abdominal CT and high CEA levels support primary CRC. Also in lymph node biopsy there was primitive adenoid cells inside undifferentiated tumor cells. Eventhough CEA level is not sufficient for CRC diagnosis; it is shown to be sensitive in studies compared with healthy people.19
Pituitary metastasis treatment is usually conservative and palliative. Because when pituitary metastasis is shown, the disease is usually in terminal stage. Surgical removel, radiotherapy and systemic chemotherapy can be applied in appropriate patients. In conservative treatment deficient hormonal replacement and as in our patient, steroid therapy for antiedema effect in cerebral metastasis is appropriate. Despite all these treatments, life expectancy is to short as 6-22 months; in stalk involvement this time is 2-4 months.16,20 Our patient was died in a short time period despite conservative therapy because of additional comorbidities.
As a result in HONKC cases, seen in type 2 DM patients in younger ages without precipitating factors, additional risk factors and comorbidities should be thought. Particularly, in persistent polyuria and sustained hypernatremia DI should come to mind. Also in metastatic malignancies with polyuria and polydipsia pituitary metastasis shuould be suspected.
None.
Author declares that there is no conflict of interest.

©2015 Dogansen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.