Journal of
eISSN: 2374-6947


Review Article Volume 3 Issue 2
North Shore Medical Center in Salem, USA
Correspondence: Pasquale Cancelliere, Sanphy Podiatry Group, Metro West Medical center, Italy
Received: January 29, 2016 | Published: March 9, 2016
Citation: Cancelliere P. A review of the pathophysiology and clinical sequelae of diabetic polyneuropathy in the feet. J Diabetes Metab Disord Control. 2016;3(2):21-24. DOI: 10.15406/jdmdc.2016.03.00062
Diabetic neuropathy (DN), affects approximately 50% of the people who have diabetes mellitus. It is also the ubiquitous aspect of diabetes mellitus that is a precursor to decreased quality of life and limb threatening complications from diabetes. The vast majority of patients who develop a diabetic foot ulcer (DFU) are neuropathic and all patients who develop Charcot Arthropathy are profoundly neuropathic. Neuropathy can range from hypoesthetic (decreased sensation) to anesthetic to painful. Painful neuropathy is typically associated with small fiber neuropathy and affects approximately 20% percent of patient with neuropathy and will dramatically affect patient daily quality of life. However, hyopesthetic neuropathy is the leading cause of ulceration, Charcot Arthopathy and amputation. This article aims at reviewing the epidemiological relevance of DN as well as its pathophysiology and a brief review of diagnosis, and assessment as well as its sequelae.
DN, Diabetic neuropathy; DFU, diabetic foot ulcer; IWGDF, international working group on diabetic foot; DPN, diabetic peripheral neuropathy; ANS, autonomic nervous system; AGE, advanced glycation end products; AENS, american extremity nerve surgery association; DSPN, diabetic sensorimotor polyneuropathy
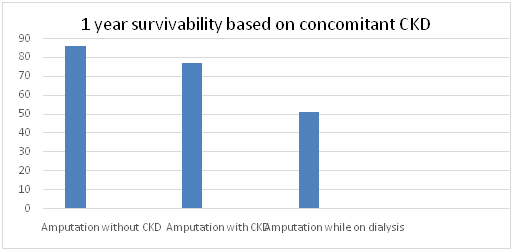
Diabetes affects 382 million people worldwide and its prevalence is expected to rise to 592 million within the next 19 years. Specifically, the International Working Group on Diabetic Foot (IWGDF), it is estimated that in 2013 approximately 382 million people have diabetes – 8.3% of the world’s population. Around 80% of these people live in developing countries. By 2030, the global estimate is expected to rise to over 552 million - 9.9 % of the adult population. Every 20 seconds a lower limb is lost to diabetes somewhere in the world. This indicates an impending pandemic of this disease process which combined with more and more sedentary lifestyle and diet habits is expected to worsen.1,2 Diabetic Neuropathy is estimated to affect nearly 50% of patient with diabetes mellitus and is associated with significant increase in morbidity and mortality. Particularly, the onset of a diabetic foot ulcer is a pre cursor to limb loss and early death. Up to 85% of lower limb amputations in diabetic patients are preceded by a foot ulcer.3 Diabetes related amputations result yield a 5 year mortality rate of 39% to 68% with a below knee amputation, and 89% with a single above knee amputation.4,5 Patients with a previous below knee amputation have a 42% chance of a contra lateral same level amputation within 1-3 years and 56% chance at 3 to 5 years. Patients with bilateral below knee amputations have a 80% mortality rate at 2 years.6,7 Furthermore, if the patient concomitantly suffers from chronic kidney disease, the mortality rate status post lower limb amputation is far more grim. One year survival rates for a single limb, below knee amputation was approximately 50% versus 76.6 and 85% in patient without kidney disease with chronic kidney disease and patient on kidney replacement therapy.8,9
One study by Brownrigg, the crude mortality rates in the groups with or without diabetic foot were 27.0% and 6.4% respectively.10 Several mechanisms may explain the link between DFU and increased overall mortality. Active ulceration has been shown to cause numerous biologic responses most notably chronic inflammation, which has been shown to play a role in the development and progression of atherosclerosis. Approximately 15% of all diabetic patients will develop an ulcer in their lifetime.11
Neuropathy is the leading causative factor in developing an ulcer, whereas ischemia will predict the outcome of infection. In a study by Reiber GE et al. in 1999 showed that diabetic foot ulcers were caused by peripheral sensory neuropathy in 63% of the cases12 (Figure 1).
Clinical presentation
Diabetic Peripheral Neuropathy (DPN) is one of three main diabetic microvascular complications along retinopathy and nephropathy, the consequences of which have been reviewed in the introduction of this paper. DPN can be categorized in 3 main types: sensory neuropathy, motor neuropathy and autonomic neuropathy. DPN is a disease whose symptoms are related to dysfunction of peripheral nerves, such as demyelination, axonal atrophy, blunted regenerative potential, and loss of peripheral nerve fibers that occur specifically in patients with diabetes.13
Classically, DPN has been described clinically as a “gloves and stockings” distribution and the symptoms are often perceived to exacerbate during the night, and in severe cases, prevent the patients from being able to sleep, further depressing their quality of life. However, a retrospective study be Rader AJ, showed that the “gloves and stockings” theory might not be completely accurate, and suggests the presentation of DPN might more metabolic than anatomic.14 However, typically, in the case of a metabolic DPN, the clinical presentation will be symmetric between limbs. In the case of asymmetric DPN, the physician should investigate more an entrapment type etiology. According the American Extremity Nerve Surgery Association (AENS) clinical guidelines of 2014, evidence indicates that using nerve decompression will minimize neuropathic DFU recurrence by over 80%. There is also evidence that nerve decompression is protective against initial primary DFU in advanced DSPN in Tinel-positive patients. Therefore, consideration of using nerve decompression to protect against recurring DFU and progression to amputation is warranted. Evidence of improved transcutaneous oxygen levels post-nerve decompression may mean that less severe neuroischemic DFU cases can also be protected.15,16-20
The AENS finds evidence that nerve entrapments so frequently found in diabetes more often represent single or multiple metabolically induced nerve trunk entrapments in areas of fibro-osseous anatomic tunnels.21 Neuropathy can range from initial stage symptoms such as “tingling” and “burning sensations, to profound neuropathy, perceived as total loss of sensation to the feet and/or hands, defined as Hypoesthesia or Anesthesia. It is these two phases of neuropathy which the foot specialist should be more aware of and remain keen on during the physical examination. These two phases will be often “minimized” by the patient or neglected during the narration of the “history of present illness” by the patient as the presenting symptoms are diminished. Also, when the patient transitions to late or advanced DPN, they may have the false perception of the neuropathy improvement as the symptoms subside.
However, Hypoesthestic DPN, as defined by Rogers and Rayaz, is the single most important risk factor for neuropathic ulceration and amputation.3,22,23 In the final stages, patient develop altered gait patterns which lead to stress fractures, ulcerations and Charcot Arthropathy, which all to commonly will lead to limb amputation and death. This review will review the causes of neuropathy and its consequences in order to sensitize the physician to assessing neuropathy accurately and early with the ultimate purpose being, prevention when possible, and early effective treatment in order to avoid progression and complications.
Anatomy of pain
Pain is the body’s perception of actual damage, injury, or simply a stressful change in the nerve’s environment. Sensory afferent nerves carry sensations from the skin, joints, viscera via large and small fibers.
When performing a focused neurological examination on the foot, each test will assess a specific set of nerve fibers which will distinguish the neuropathy in “small” or “large” fiber neuropathy and be able to grade the severity of the neuropathy itself. This is a critical assessment skill in deciding which patient is at higher risk for limb threatening diabetic foot complications.
A nerve may be sensory, motor or sensory-motor (mixed). There are three types of nerve fibers in a mixed nerve that include: Sensory nerve fibers (afferent fibers), Motor nerve fibers (efferent fibers), and Autonomic nerve fibers (autonomic fibers). There are three types of peripheral nerve fibers based on their diameter: A group, B group, C group. Large A-delta myelinated fibers and small C unmyelinated fibers are mainly responsible for carrying nociceptive sensations. Superficial and lancinating pain is usually associated with A fibers.
Fibers of the A group have a large diameter, are myelinated, and have the highest conduction velocity of all the nerves in the body. The A group consists of four types of nerve fibers: A alpha fibers (afferent or efferent fibers; A beta fibers (afferent or efferent fibers); A gamma fibers (efferent fibers); A delta fibers (afferent fibers).
A alpha fibers (Ia fiber or Ib fibers) are characterized by high conduction velocity; Ia fibers are related to muscle spindle primary endings (muscle sense) Ib fibers are related to golgi tendon organs (muscle sense);A beta fibers (II fibers) carry sensory information related to muscle spindle secondary endings, touch, and kinesthesia. A delta fibers (III fibers) carry sensory information related to pain and cold temperature.
Alpha fibers are also responsible for vibration sensation, which is typically the first sensation that is lost in DPN. Loss of vibration sensation is defined as the inability to perceive the stimulus of a 128 m Hz tuning fork tapped with the thinner eminence of the physician and places against a joint of the foot, typically the 1st metatarsal phalangeal joint. In a study of 216 patients by Deribe B. et al.24 patients with loss of vibration sensation were 3.91 times more likely to develop a diabetic foot ulcer.24 The author of this article performs this part of the diabetic foot exam as the first part of the neurologic examination of the diabetic patient, prior to performing the vascular part of the exam. Semmes Weinstein Monofilament testing, which tests touch, or “protective” sensation, has been found to be poorly sensitive in the clinical experience of the author and therefore not as a reliable as a predictor for staging DPN.
The B group Nerve fibers are myelinated with a small diameter. Generally, they are the preganglionic fibers of the autonomic nervous system and have a low conduction velocity.
The C group fibers are unmyelinated and as the B group fibers have a small diameter and low conduction velocity. These fibers include: Postganglionic fibers in the autonomic nervous system (ANS). C fibers use substance P as a neurotransmitter. Secondly, they include nerve fibers at the dorsal roots. These fibers carry the following sensory information: Nociception (pain); Temperature; Touch; Pressure; Itch. A deep seated, burning, itching, aching type pain is often accompanied with hyperalgesia and allodynia and is transmitted via slow, unmyelinated C fibers.
DPM is essentially a demyelinating process where the large myelinated nerve fibers will be affected initially. Typically, C fibers, will be affected last and this is confirmed clinically that by the patient presents with impaired proprioception, they have lost all other sensory characteristics, and therefore places them at the highest risk. As a general rule, large fiber neuropathy is characterized by painless paresthesia with impairment of vibration, joint position and touch and pressure sensation (“protective sensation”), and loss of Achilles Tendon reflex. In advanced stages of DPN, sensory ataxia can occur. Large fiber neuropathy results in slowing of nerve conduction.
Small fiber neuropathy is associated with burning, pain, and impairment of pain and temperature sensations, which are often associated with autonomic neuropathy. Nerve conduction studies are usually normal but quantitative sensory and autonomic tests are abnormal. Small fiber neuropathy results in increased morbidity and mortality, but autonomic neuropathy in diabetes, does not occur without sensory motor neuropathy.25 This is very much consistent with the clinical pattern seen in the classic “intrinsic minus” foot type and Charcot Arthropathy.
Pathophysiology of DPN
Four major pathways are typically attributed to the development of DPN:
However, these all represent “metabolic” aberrations of nerve physiology.
The Polyol focuses on the enzyme aldose reductase. This enzyme normally serves the purpose of reducing toxic aldehydes in the less to inactive alcohols, but when glucose concentration in the cell becomes too high, aldose reductase that glucose into sorbitol, which is later oxidized into fructose. In this process, the aldose reductase consumes the cofactor NADPH, which essential in generating gluthathione, which is critical in reducing intracellular oxidative stress.27 In a study performed by Engerman et al.28 diabetic dogs were treated for 5 years with an aldose reductase inhibitor, and the nerve conduction velocity as it does in diabetic patients. When the treatment was stopped, the diabetes induced defect in nerve conduction velocity was prevented.28
Intracellular Production of AGE precursor’s damage cells on the other hand in 3 separate mechanisms. The first mechanism involves the endothelial cell. It modifies intracellular proteins including proteins involved in gene transcription. The second mechanism is that the AGE will diffuse out of the cell and modify extracellular matrix molecules nearby which changes the signaling between the matrix and the cells and cause cellular dysfunction. This pathway can be seen in the cross linking of collagen and consequent tendon and ligament pathology. The final mechanism is that AGE products diffuse out of the cell and modify circulating proteins in the blood such as albumin. The proteins will then activate AGE’s causing the production and release of inflammatory cytokines and growth factors which in turn lead to vascular pathology.29
The Protein Kinase C activation is precipitated when hyperglycemia increases the synthesis of diacylglycerol, which activates the cofactors for protein kinase C. PKC will have effects on gene expression such as downregulation of endothelial nitric oxide (NO), and upregulation of vasoconstrictor endothelin-1. This will result in changes to Schwann cell metabolism and ultimately axonal flow.
The final pathway precipitated by hyperglycemia is the hexosamine pathway. When intracellular glucose is high, glucose is metabolized thru glycolysis. Some of the fructose 6-phosphate gets diverted into a signaling pathway in which an enzyme called GFAT converts it into UDP (uridine phosphate) N-acetyl glucosamine. As an end stage, this binds to serine and threonine which will lead in changes to gene expression.29
The ubiquitous factors however for development of DPN remain hyperglycemia an reactive oxygen species, also called “free radicals”, which are directly neurotoxic. The research done by Du XL et al, and Nishikawa T., showed that a differentiating feature common to all cell types damaged by hyperglycemia is an increased production of reactive oxygen species. Intracellular hyperglycemia, more glucose oxidized in the TCA, which pushes more electron donors into the electron transport chain. This, the voltage gradient across mitochondrial membrane increases until a critical threshold is reached and electrons back up to coenzyme Q which donates electrons one a time to molecular oxygen, thereby generating superoxide.29
Diabetic Polyneuropathy continues to be a common and serious complications of diabetes mellitus. It affects approximately 50% of the patient with diabetes mellitus and overall, approximately 20% of the neuropathy is painful, meaning it is a small fiber neuropathy. The prevalence of DPN increases with duration of the diabetes. In a study by Pirart J et al.29 the incidence of neuropathy increased from 7.5% on initial presentation to 50% at 25 years follow up.29
In its initial stages, it will present with the common “tingling”, “burning”, and other changes in perception of temperature. These symptoms are classified as allodynia. As the disease process progresses the symptoms will changes and progress eventually to complete loss of sensation, known as anesthesia. Sadly, DPN is a progressive and degenerative disease process that targets sensory, motor and autonomic nerve pathways. Typically sensory neuropathy is the first stage of DPN, and if not treated appropriately and is strict blood glucose control is not implemented, it will precipitate to motor and autonomic. Motor neuropathy will lead to loss of intrinsic muscle innervation causing different foot deformities. The foot deformities can range from slight contracture of the proximal inter phalangeal joints causing the classic “hammer toe” deformity”, to more devastating deformities which destroy the anatomic architecture of the foot, such as Charcot Arthropathy. Foot deformities will result in abnormal pressure distribution which will invariably lead to ulcer formation. Autonomic neuropathy will lead to skin changes associated with altered cutaneous blood flow and loss of normal function of sweat and oil glands, creating skin that is dry and fragile like in the case of callous. Although a previous ulceration is the most reliable predictor of a new ulcer, the presence of a callous has been found highly predictive in several studies. It is well published in the literature that the development of a diabetic foot ulcer is the single most common precursor to limb amputation. Early recognition, thru appropriate examination techniques, and history taking, coupled with rapid and aggressive treatment is paramount for arresting the natural progression of DPN. Semmes Weinstein Monofilament testing, Vibratory Tuning Fork testing, 2 point discrimination testing, Pressure Specified Sensory Device, as well as Epidermal Nerve Density Biopsies and EMG and NCV’s are all necessary components of the focused neurological exam. Treatment options range from pharmacologic intervention, to surgical such as neurolysis as advocated by the AENS. In its 14-year history AENS has seen nerve decompression produce dramatic clinical benefit in diabetic peripheral neuropathy patients. The clinical and laboratory evidence strongly indicates the frequent nerve entrapments seen in diabetic sensorimotor polyneuropathy (DSPN) are a secondary pathology which frequently accompanies DSPN, is responsible for many serious complications which are frequently responsive to safe and effective surgical neurolysis. Nerve decompression surgery has been found to produce balance improvements, which may aid in fall prevention, decreased ulcer formation and recurrences, improvements in pain, and recovery of protective sensation.15 If pharmacologic and other non invasive techniques fail to alleviate the symptoms and progression of DPN, a prompt referral to a physician who performs competent execution of these procedures is a must to avoid catastrophic and too often irreversible effects of DPN.None.
Author declares that there is no conflict of interest.

©2016 Cancelliere. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.