Journal of
eISSN: 2374-6947


Research Article Volume 5 Issue 6
Laboratory of Physiology, Pharmacology and Pharmacopoeia, UFR-Science of Nature, University in Abidjan, Côte d’Ivoire
Correspondence: Monteomo Gnate Francois, Laboratory of Physiology, Pharmacology and Pharmacopoeia, UFR-Science of Nature, University in Abidjan, Côte d’Ivoire
Received: November 21, 2018 | Published: December 10, 2018
Citation: Monteomo GF, Kamagate A, Gnangoran BN,et al. Risk factors associated with metabolic syndrome by enriched food in pig fat in the Wistar rats. J Diabetes Metab Disord Control. 2018;5(6):216-220. DOI: 10.15406/jdmdc.2018.05.00169
Background: The metabolic syndrome is a state of morbidity characterized by factors such as abdominal obesity, high blood pressure, hyperglycemia, low HDL cholesterol (HDL-C) and high triglyceride levels. The objective of this study was to evaluate the effect of a diet supplemented with pig fat 10% and 30% on the occurrence of at least three of the five factors that characterize metabolic syndrome with a model animal imitating the pathological state of man.
Material and methods: The experiment was conducted on three groups of Wistar rats (Rattus norvegicus) ad libitum consumption pig high-fat diet (HF, 30%), pig lower-fat diet (LF, 10%) and normal diet (ND) for 9 weeks. At the end of the experiment, metabolic parameters were obtained and adipose tissue was weighted.
Results: The results showed that total cholesterol was significantly increased in rats consuming 10% and 30% fat compared to control rats (142.44±5.3 and 148.86± 4.25 vs 136.02±4.8mg/dl; p < 0.05;n=7). It’s the same for blood triglycerides concentration in experimental rats (LF, HF) and control rats with values of 128.25±4.7 and 129.02±5.4 vs 112,4±3.6mg/dl (p<0.05). The LDL cholesterol was singularly high in rats fed the 30% HF diet compared to the rest of the animals (112.8±8.5 vs 96.40±9.3 and 90.4±4.2 mg/dl;p<0.05). The serum fraction of HDL cholesterol was significantly lower in the experimental rats (LF, HF) than the control rats (61±4.9 and 54.97±5.9 vs 93.5±4.1 mg/dl;p<0.05). The atherogenic indices CT/HDL and LDL/HDL were significantly higher in LF and HF rats compared to control rats (1.78 and 1.52 vs 1.34;p<0.05). The plasma glucose level of the experimental rats (LF, HF) was significantly reduced compared to that of the control rats (23.3±3.21 and 25.7±2.4 vs 34.7±3.04mg/dl) (p<0, 05). The percentage of epididymal adipose tissue is also greater in diet animals (HF) than standard (RS) and experimental (LF) rats (1.4±0.30% vs. 0.79±0, 06% and 0.75±0.04%;p <0.05)
Conclusion: Elevated levels serum of total cholesterol, LDL cholesterol and atherogenic indices (TC/HDL and LDL/HDL), low HDL-C and excessive accumulation of adipose tissue (abdominal and epidydimal) have shown that the standard diet supplemented with 10% or 30% of pig fat caused a metabolic syndrome. Like the animal fat, incorporation at high doses in the diet could affect health because of the presence of hyperLDLlemia found in our test which one were the main risk factors for cardiovascular disease.
Keywords: Biochimicals parameters, Fat diet, Adipose tissue, Metabolism syndrome, Wistar rat
Metabolic syndrome (SM) is also known as Syndrome X,1 is a morbid condition, characterized by an aggregate of cardiovascular risk factors and type 2 diabetes such as abdominal obesity, high blood pressure, high blood sugar, low HDL-cholesterol (HDL-C) and hypertriglyceridemia.2 A person has metabolic syndrome (MS) when he/she has a combination of 3 or more specific health risks. It’s becoming an emerging global public health problem, global prevalence can be estimated at about a quarter of the world's population. In other words, more than one billion people worldwide are affected by the metabolic syndrome.3 This epidemic affects about 35% and 50% of the adult population respectively to United States and Northern Europe.4 The prevalence of MS varies by 4.4 % in Côte d'Ivoire.5 The deleterious effects of MS draw research efforts in developing new interventions to reduce its burden on the healthcare system. Due to its multifactorial nature, selecting an adequate experimental model that best represents the path physiology of MS in humans can be rather challenging. Rats and mice are the most common animal models used in investigating MS. Some of the various approaches used to induce MS in rodents include dietary manipulation, genetic modification and drugs.6 Although heredity is one of the causes of this syndrome, the vast majority of cases is rather related to a sedentary lifestyle and a poor diet including the consumption of high carbohydrate and fat food that would cause lipid abnormalities which are atherogenic.7
Thus, several studies use diets enriched with fats of various origin for the induction of the metabolic syndrome in animal models developing symptoms as close as possible to those found in humans to test appropriate treatments.8,9 Among these, they are high fat diets lardy based (thick fat layer of subcutaneous tissue of pig) appears to be the most obesogenic with a composition predefined.10 Pig’s fat is a melting fat that is characterized by a high content of saturated fatty acids (40 to 50%) and a low concentration of linoleic acid (from 2 to 8%).
In addition, previous work done in our laboratory showed that a consumption of granules (FACI®, Côte d’Ivoire) supplemented by palm oil to 15% didn’t both lead to weight gain and elevated cholesterol levels in Wistar rats. Of even, an absence the metabolism syndrome has been noted in these animals aged 8 to 9 after 4 weeks of testing.11
The objective of this work is to evaluate the effects of a normal diet supplemented with low dose of 10% (LF) and high dose of 30% (HF) pig fat on the appearance of metabolic syndrome factors in Wistar rats during a period of 9 weeks.
Animal material and livestock
The experimental protocol consisted of 21 rats mixed in 3 groups of 7, as well as Wistar strain (Rattus norvegicus) composed of males and females, of the pet shop of the physiology, pharmacology and pharmacopoeia laboratory of the UFR-SN, Nangui Abrogoua University, Abidjan. Rats aged 8 to 9 weeks and with a homogeneous mean body weight of 110.60±7.5 g are fed either with pellets (FACI®, Abidjan, Côte d’Ivoire) or to grains added to 10% (LF) and 30% (HF) pig fat for experimental rats for 9 weeks (63 days). The breeding is done in a lighted room 12 hours a day, and whose temperature is kept constant (22 to 23°C) in an enclosure equipped with an air conditioner (Smart, Canada). The animals have free access to food and water and are weighed once a week.
Technical material
Technical material consists of electronic scale, reagent (alcohol, ether), anesthesia bell, cotton, Pasteur pipette, dry tubes and specimens.
Collection of blood samples
Blood samples were taken in healthy rats on days 0 and 56. At each sample, the animals were fasted for 12 hours. In animals anesthetized with ether, blood was taken from the retro-orbital sinus using a sterile Pasteur pipette. Blood samples were collected in dry tubes. After centrifugation at 4000rpm for 20min, the serum is recovered for biochemical analyzes (triglycerides, total cholesterol, and HDL-cholesterol and blood glucose).
Biochemical analyzes
Total cholesterol (TC), HDL-cholesterol (HDL-C), triglycerides (TG) and blood glucose are determined by the enzymatic and colorimetric method,12 using a Robonik Prietest (India). As for LDL-cholesterol (LDL-C), it’s calculated from the Friedwald formula below: LDL-C = CT - (HDL-C + TG) / 5
These determined biochemical parameters expressed (mg/dl) make it possible to calculate an atherogenicity index (CT/HDL or LDL/HDL) which is a revealing indicator of the arterial and especially coronary risk.13
Statistical analyzes
The statistical analysis of the data is done with the Graph Pad Prism 5.01 software (San Diego, California, USA). The results are given as an average followed by the standard error on the mean (M±S EM). The analysis of the variance is completed by the Turkey-Kramer test to compare the average values of the different parameters. Differences are considered significant in P<0.05.
After 9 weeks, the body weight gain (g) of LF rats was significantly higher than those ND rats and HF rats (59.5±6.8g vs 26.9±7.3g and 30.8±4.6g;P<0, 05) (Figure 1A) .
Similarly, body weight gain in the animal correlated with the amount of food ingested (g/d). Thus, the LF rats have consumed an average of 42, 75±5,7g/day while those of the normal group (ND) and experimental (HF) consumed respectively, 38.5±25 and 27.5±3g/day (Figure 1B).
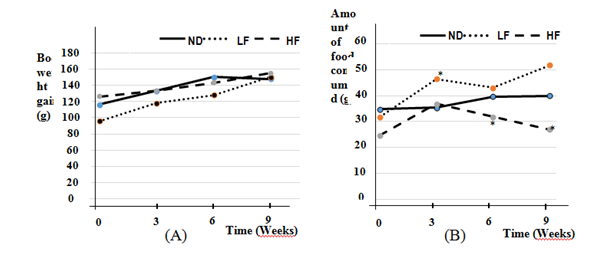
Figure 1Body weight (A) and amount of food consumed (B) in Wistar rats fed food supplemented with 10% (LF) and 30% (HF) pig fat during 9 weeks. The data are presented on average±SEM (n=7 rats; P<0.05).* Significative difference between Normal diet (ND) and diet groups supplemented with 10% (LF) or 30% (HF) pig fat.
The average weight relative adipose tissue (abdominal and epididymal) have been reported in percentage (%) of body weight of rats following the animals dissection at the end of the experiment. The percentage of abdominal adipose tissue of HF rats was significantly higher than rats receiving normal (ND) and experimental LF diet (3.5±0.9% vs 2.9±0.6% and 1.8±0.7%; p<0.05). The percentage of epididymal adipose tissue was also greater in HFrat than Normal (ND) and experimental (LF) rats (1.4±0.30% vs 0.79±0, 06% and 0.75±0.04%;p<0.05) (Figs. 2 and 3). However, perirenal adipose tissue didn’t show a significant difference between all groups of rats (0.8±0.05% vs 0.7±0.08% and 0.75±0.04%; p>0.05).
The levels of triglycerides and lipoproteins (CT, LDL-C, and HDL-C) associated with their atherogenic index and blood glucose was recorded in the table above.
The consumption of the normal food supplemented with pig fat had various effects on metabolic syndrome components such as the body weight gain, adipose tissue, blood lipids associated to their atherogenic indices and metabolism carbohydrate.
The significant increase in body weight gain of LF rats (p<0.05; n=7) compared to other animals over a period of 9 weeks would be due to palatability high for this fat diet that has been relatively moderate. The underperformance of body weight gain in rats HF be explained by having a resistance to obesity following an adaptation of animals to fatty diets or a reduction of food consumption (Figure 1B).14
In addition, the significant increase in the percentage of epididymal and abdominal adipose tissue was observed in HF rats compared to the rest of the rats (p<0.05) (Figure 2 & Figure 3) confirmed an abnormal development of adipose tissue synonymous with obesity, more predictive of metabolic syndrome in these animals although their body weight is not known to increase significantly.
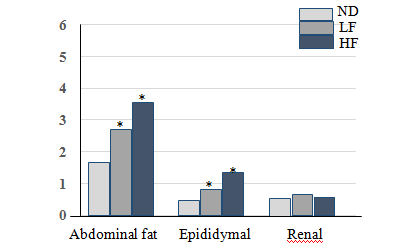
Figure 2Abdominaux fats, epididymal and renal adipose tissue in Wistar rats at the end of the diet. The data are presented as mean±SD (n=7 rats, P<0.05) *: Significant difference between Normal diet (ND) and diet groups supplemented with 10% (LF) and 30% (HF) pig fat.
The analysis of blood lipid composition showed that total cholesterol levels, LDL cholesterol and increased triglycerides levels very significantly in the experimental rats (HF and LF) compared to control rats (p<0.05; n=7) (Table). In addition, the LDL cholesterol was singularly in HF rats. Indeed, the increase in triglyceride levels and in particular LDL-C is a tool for assessing and monitoring the presence of the risk of coronary disease and atherosclerosis.15 The alteration of lipid metabolism already emphasized has also been found in other experiments with the fat doses,16 and were the main cause of MS regardless of obesity.
|
Initial concentrations (D0) |
Final concentrations (D 63) |
||||
|---|---|---|---|---|---|---|
Physiological parameters |
ND |
LF (10%) |
HF (30%) |
ND |
LF (10%) |
HF (30%) |
TC, mg/dl |
134,2 ± 1.4 |
131,5 ± 1.66 |
138,17 ± 1.48 |
136,02 ± 4.8 |
142.44 ± 5.3 * |
148.86 ± 4.25 * |
HDL-C, mg/dl |
90,5 ± 5.4 |
78.5 ± 5.7 |
91.1 ± 4,8 |
93,5 ± 4.1 |
61 ± 4, 9 |
54, 97 ± 5.9 * |
LDL-C, mg/dl |
92.5 ± 4.7 |
90.1 ± 4,1 |
79.5 ± 4,3 |
90,4 ± 4,2 |
96.40 ± 9.3 * |
112.8 ± 8.5 * |
TG, mg/dl |
113.6 ± 2.6 |
109.8 ± 1.8 |
115,25 ± 2.1 |
112.4 ± 3,6 |
128.25 ± 4,7 * |
129.02 ± 5.4 * |
Ai = TG/ HDL-C |
1.25 ± 0.48 |
1.39 ± 0.3 |
1.26 ± 0.55 |
1.20 ± 0.85 |
2.09 ± 0.95 * |
2.38 ± 0.91* |
Ai = LDL/ HDL-C |
1,02 ± 0,8 |
1,15 ± 0,71 |
0.86 ± 0, 8 |
0.95 ± 0, 61 |
1.57 ± 0.55 * |
2.07 ± 0.55 * |
Glucose, mg/dl |
33.7 ± 1.33 |
35.1 ± 1.05 |
31.9 ± 1.12 |
34.7 ± 3.04 |
23.3 ± 3.21 * |
25.7 ± 2.4 * |
Table 1 Biochemical Parameters are Wistar rats fed various diets for 9 weeks. The data are presented as mean ± SD (n=7 rats, P<0.05) *: Significant difference between supplemented diet groups and Control group. HF, high fat diet; LF, low fat diet; ND, Normal diet (Control group). LDL-C, low-density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; CT, total cholesterol; TG, triglycerides; Ai, Atherogenic index.
The mechanism of lipid metabolism dysfunction in the advent of the MS begins with the increase of the size; the number and the lipolytic activity of the adipocytes are at the origin of a significant release of triglycerides from the adipose tissue resulting at high levels of circulating fatty acids. Also, the presence of fat abdominal and epididymal fat (figure 3) that was reported in this experiment could aggravate the effects of excess adiposity by promoting a greater influx of fatty acids directly to the liver. This leads an increase in synthesis hepatic triglycerides and VLDL lipoproteins production and leads to long-time dyslipidemia,17 a sign of profound disturbance of lipid metabolism.
Conversely, the HDL cholesterol level was significantly higher in control rats than LF and HF rats (93.5±4.1 vs 61±4.9 et 54.5±5.9 mg/dl) in the table. This has been also noticed in rats fed a diet enriched with both animal and vegetable fat, giving a greater drop in HDL-C (55, 99±3.3 vs 27.72±2mg/dl),18 which will increased the risk of metabolic syndrome in animal11 and in humans.19 Indeed, HDL plays an important role in the reverse transport of cholesterol from peripheral tissues to the liver,20 but also exert direct actions on the vascular endothelium with, in particular, anti-inflammatory and antioxidant effects,21 delaying the onset of symptoms of metabolic syndrome as showed the animals receiving the normal diet.
The atherogenic index TC/HDL and LDL/HDL were significantly greater in HF and LF rats compared to control group (1.78 and 1.52vs. 1.34;p<0,05), this is predictable the atherosclerosis.18,22 These atherogenic index to experimental rats are nevertheless lower at 5.6 obtained with a diet supplemented both fat and sugar,22 which involves high fatty acid by the mechanism already described.
In sum concerning cholesterol parameters, elevated serum TC, LDL-C and atherogenic index, low level of HDL-C and obesity were considered as a major risk factor for the development of the metabolic syndrome following chronic consumption of hyperlipidic diet in rodents and human.1,8,9
In terms of carbohydrate metabolism, against all odds, this hyperlipidic diet leads to a clear reduction in plasma glucose levels in LF and HF rats compared to control rats (23.3±3.21 and 25.7±2.4 vs 34±3.04mg/dl;p<0.05).23 This could be attributed to a deficiency of glucose 6 phosphatase and the blocking of glycogenolysis then hepatic neoglucogenesis at the origin of moderate hypoglycaemia.24 This abnormal reduction in blood glucose occurs when there is a risk of insulin resistance and it could be the cause of metabolic dyslipidemia previously noted.25
The use of a diet supplemented with 10% pig fat in 9-week-old Wistar rats revealed three risk factors leading to the metabolic syndrome based on hypertriglyceridemia, hypercholesterolemia and low HDL. The remarkable increase of LDL in the animals subjected to the 30% pig fat in diet confirms at this dose its more atherogenic character compared to that 10%. Added to this is a disruption of carbohydrate metabolism in animals with moderate hypoglycaemia. These results support the epidemiological data that suggest that a high fat diet promotes the development of the metabolic syndrome.
The author would like to thank the Masters students and doctoral of Laboratory of Physiology, Pharmacology and Pharmacopoeia, Nangui Abrogoua University to provide assistance to testing on laboratory animals.
The author declares that there is no conflict interest.

©2018 Monteomo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.