Journal of
eISSN: 2374-6947


Type 1 diabetes (T1D) is a chronic autoimmune disease in which an imbalance between pro- and anti-inflammatory and/or regulatory cytokines leads to T1D development. That prompted us to study the meaning of plasma constituents of the immune response in BB/OK rats developing T1D and 2 congenic derivatives without T1D (BB.1K; BB.4S) or with diminished T1D frequency (BB.6S). All animals were studied at an age of 4weeks, some days before initial insulitis is discovered in BB/OK rats. Plasma concentrations of 24 biological markers were assayed using the Bio-Plex Pro Rat cytokine 24plex assay system (order no.171-K1001M; Bio-Rad, Hercules, CA, USA).
Significant differences between BB/OK and all other strains were only observed for M-Csf. BB/OK rats had significantly higher values than BB.6S, BB.4S and BB.1K rats. In addition, BB.6S rats had significantly reduced M-Csf values compared to BB.1K rats. Obviously significant differences were found in IL-18 and Epo, where samples from BB.6S had the highest values vs all other strains.
M-Csf controls the production, differentiation and function of macrophages. They are the first cells to invade the pancreatic Langerhans islets before T1D onset. The correlation of M-Csf concentration in plasma with T1D development in BB/OK rats and their congenic derivatives may provide evidence that macrophage production is activated in dependence on T1D development at an early age.
Keywords: type 1 diabetes, diabetes-prone bb rats, diabetes-resistant congenic bb rats, cytokines, m-CSF, il-18, erythropoietin
BB, bio breeding; BB/OK, bio breeding/ottawa karlsburg; BB.6S, BB.4S and BB.1K, congenic derivates of bb/ok with transferred chromosomal region from SHR or KWR; SHR, spontaneously hypertensive rats (SBP 180-240mm hg); KWR, inbred wild rats captured in northern germany; T1D, type 1 diabetes; MHC, major histocompatibility complex; IDDM1/Iddm1, insulin-dependent diabetes mellitus QTL1; Iddm2-Lyp, insulin-dependent diabetes mellitus QTL2-lymphopenia; Gimap5, gtpase imap family member 5; IL-1RA, il-1 receptor antagonist; IL-1a, -1ß, -2, -4, -5, -6, -10, -12p70, -13, -7, -17a, and -18, interleukins 1a 18; Gm-Csf, granulocyte-macrophage colony-stimulating factor; G-Csf, granulocyte colony-stimulating factor; M-Csf, macrophage colony-stimulating factor; Cxcl1, chemokine (C-X-C) ligand 1; Ifng, interferon gamma; Tnfa, tumor necrosis factor alpha; Epo, erythropoietin; Ccl2, Ccl3, Ccl5 and Ccl20, chemokine (C-C motif ) ligands 2, 3, 5 and 20; Vegf, vascular endothelial growth factor; Th1 and Th2. T helper 1 or 2
Type 1 diabetes (T1D) is a chronic autoimmune disease in which a loss of tolerance to insulin-producing ß-cells in the pancreatic islets results in impaired glucose homeostasis. T1D is characterized by a destruction of pancreatic ß-cells by islet-reactive T cells. Proinflammatory cytokines such as IL-1β, IFN-γ and TNF-α promote the destruction of pancreatic ß-cells, while regulatory cytokines (IL-10 and TGF-β1) and anti-inflammatory cytokines (IL-1 receptor antagonist [IL-1RA]) avoid ß-cell destruction. An imbalance between pro- and anti-inflammatory and/or regulatory cytokines is essential for the development of T1D.1?3
Bio Breeding (BB) rats are widely used as a model for T1D because they display many aspects of disease pathogenesis similar to that found in humans and harbor a general predisposition to autoimmunity, which is modulated by genetic background. In BB rats, the development of diabetes proceeds from an initial phase of insulitis, characterized by T and B cell infiltrates in the absence of ß-cell damage, to an aggressive stage in which ß-cells are destroyed and glucose homeostasis is disrupted.
T1D clusters in families and is frequently associated with other autoimmune disorders, suggesting that an underlying genetic susceptibility compromises tolerance to multiple normal tissues. Extensive linkage analysis of families with T1D and BB rats yielded more than 20 genetic susceptibility loci. Among these, the Major Histocompatibility Complex (MHC) class II locus exerts the most important influence on disease development termed IDDM1 in human and Iddm1 in rats. Several non-MHC related genes have also been implicated. One of them is the gene for lymphopenia in BB rats, termed Iddm2 (also known as Lyp; Gimap5 and Ian4), which – like Iddm1– is also essential for T1D in BB rats. Nonetheless, multiple additional loci remain to be identified, although characterization of these gene products has been hampered by the large number of immune defects associated with the disease and a limited understanding of the key pathogenic mechanisms.4
One of the diabetes-prone BB rat strains is the BB/OK rat, which belongs to the best-characterized BB rat subline in the world.5 In addition, several congenic BB/OK rat strains with varying diabetes frequency have been developed.5?8 To elucidate the meaning of plasma constituents of immune response, we studied 24 plasma constituents of BB/OK rats and 2 congenic derivatives without T1D development, lacking one essential diabetogenic gene Iddm1 (BB.1K;)7 or Iddm2 (BB.4S;).6 Furthermore, one congenic BB/OK strain developing T1D at a frequency of 15% (BB.6S) was used. This congenic BB/OK strain develops such a low diabetes frequency despite the fact that it possesses both essential diabetogenic genes Iddm1 and Iddm2.8 All animals were studied at an age of 4weeks, some days before first insulitis is discovered in BB/OK rats.9
Animals
All rats were bred and kept in our own animal facility under strict hygienic conditions. All rats had free access to food and acidulated water. All experiments were performed in accordance with the regulations for animal care of the Ministry of Nutrition, Agriculture and Forestry of the Government of Mecklenburg-Vorpommern (Germany). BB/OK was used as an animal model for T1D and its congenic derivatives generated with varying diabetes frequency, as shown in (Table 1).10
Strain |
Transferred |
Donor Strain |
Diabetes-Frequency (%) |
Rat Genome |
Rat Genome (%) |
BB/OK |
Parental strain |
100 |
0 |
0 |
|
BB.6S |
6q32 |
SHR |
10 |
33 |
1,3 |
BB.4S |
4q22/Lyp/Iddm2 |
SHR |
0 |
57,3 |
2,2 |
BB.1K |
20p/MHC/Iddm1 |
SHR |
0 |
3 |
0,1 |
Table 1 Whole litters of BB/OK (n=10), BB.6S(n=11), BB.4S(n=9) and BB.1K(n=6) born on the same day were used at an age of 4 weeks. Blood samples were obtained from non-fasting rats by puncturing the ophthalmic venous plexus to determine plasma constituents of the immune system
Determination of cytokines
Plasma concentrations of 24 biological markers were assayed in rats using the Bio-Plex Pro™ Rat cytokine 24-plex assay system (order no.171-K1001M; Bio-Rad, Hercules, CA, USA), which allowed simultaneous identification of cytokines in a 96-well filter plate, as described recently:11 interleukins(IL-1a, -1b, -2, -4, -5, -6, -10, -12p70, -13, -7, -17a and -18), granulocyte-macrophage colony-stimulating factor(Gm-Csf), granulocyte colony-stimulating factor(G-Csf), macrophage colony-stimulating factor(M-Csf), chemokine(C-X-C) ligand 1(Cxcl1), interferon gamma(Ifng), tumor necrosis factor alpha(Tnfa), Erythropoietin(Epo), chemokine(C-C motif) ligand 2(Ccl2), 3(Ccl3), 5(Ccl5) and 20(Ccl20) and vascular endothelial growth factor (Vegfa). The range of detection for all target cytokines was 2–32,000pg/mL.
Statistical analysis
Data are given as mean±SD. Differences were assessed by one-way analysis of variance with the Bonferroni-Holm correcting using the statistical analysis software SPSS (SPSS Inc., Chicago, IL, USA).
As shown (Figure 1), there were no significant differences between strains in terms of Il-1a, 4, 10, 17a, G-Csf, and Ccl20. Il-12p70 was only detectable in 3 samples of BB/OK rats and in no sample of the other strains, whereas Ccl3 was detectable in 2 out of 10 samples in BB/OK, in all samples of BB.6S (11/11) and in 3 out of 9 samples of BB.4S. In BB.1K samples, no Ccl3 was detectable. Significant differences between BB/OK and all other strains were only observed for M-Csf. BB/OK rats had significantly higher values than BB.6S, BB.4S and BB.1K rats. In addition, BB.6S rats had significantly reduced M-Csf values compared to BB.1K rats. Furthermore, BB/OK rats also showed significantly higher values of Il-1b, Il-5, Il-6, Il-13, and Gm-Csf than did BB.4S rats (BB/OK>BB.4S). For Il-7 and Cxcl1, BB/OK rats had higher values than did BB.6S and BB.1K (BB/OK>BB.6S and BB.1K), whereas for Infg, Tnfa, and Vegfa, BB/OK rats showed higher values than did BB.6S and BB.4S (BB/OK>BB.6S and BB.4S). In contrast to all other plasma constituents, Ccl5 is significantly increased in BB/OK and BB.4S compared to BB.6S and BB.1K. Only in Ccl2 were significant differences between BB/OK and BB.6S observed. Obviously significant differences were found in IL-18 and Epo, where samples of BB.6S had the highest values of all strains. Therefore, Epo and Il-18 are highly correlated (r=0.957; p<0.0001). In addition, there were only two significant correlations between T1D frequency and plasma concentrations for M-Csf (0.743, p<0.0001) and Vegfa (0.698, p<0.0001).
In T1D, the insulin producing ß-cells are destroyed by an imbalance between pro- and anti-inflammatory and/or regulatory cytokines. This prompted us to study the cytokine profile in the plasma of diabetes-prone BB/OK rats and three congenic strains partially (BB.6S) or totally protected (BB.4S; BB.1K) from T1D development.
As demonstrated (Figure 1), the anti-inflammatory cytokines such as Il-2, Il-4 and Il-10 indicate no preventive association with T1D, because their concentrations showed no significant differences between BB/OK and their congenic derivatives. Moreover, Infg and Tnfa as pro-inflammatory cytokines are significantly increased in BB/OK, developing to 100% T1D frequency in comparison to BB.6S and BB.4S, whose T1D frequency was 10% and 0%, but there is no significant difference between BB.1K and BB.4S, both of which did not develop T1D. Therefore it seems that the classical cytokines thought to be essential for the development of T1D do not play an important role in diabetes in these rat strains at an age of 4weeks.1 In contrast, obvious correlations were observed between T1D and M-Csf as well as Vegfa, whereas IL-18, Epo and Ccl3 were found to be very significant factors for the reduction of T1D in congenic BB.6S.
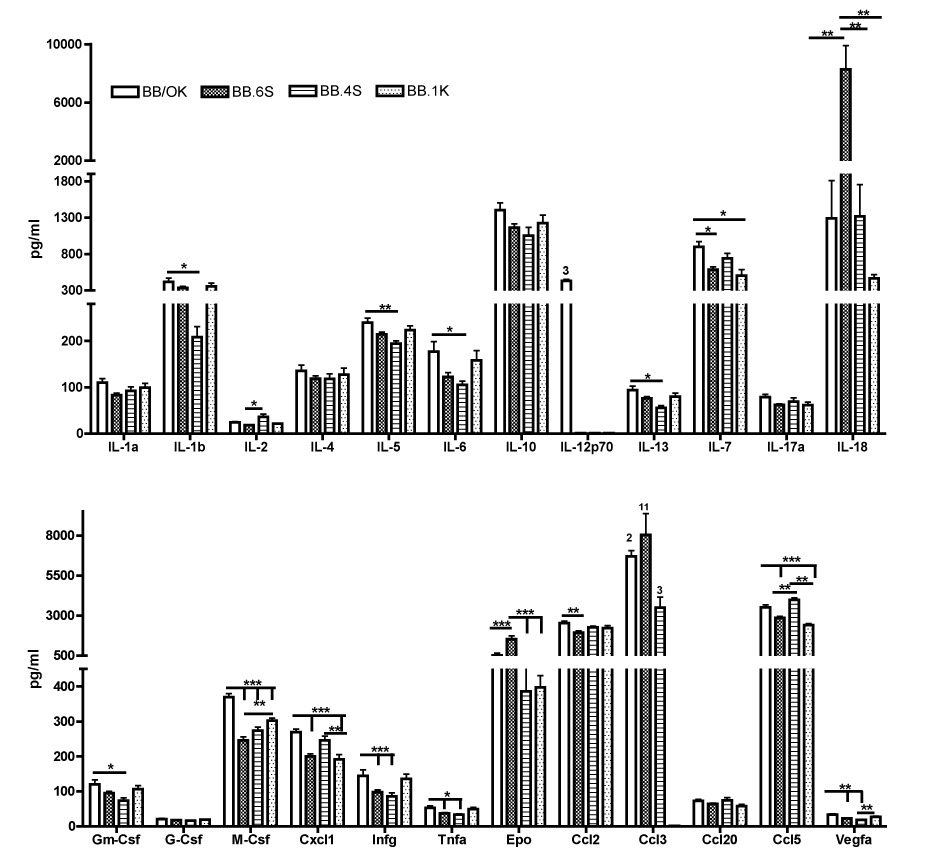
M-Csf controls the production, differentiation and function of macrophages. They are the first cells to invade pancreatic Langerhans islets before T1D onset. The correlation of M-Csf concentration in plasma with T1D development in BB/OK rats and their congenic derivatives may provide evidence that macrophage production is activated in dependence on T1D development at an early age. Because Vegfa is stimulated by M-Csf, the correlation between T1D and Vegfa is, therefore, not unexpected.12
The highest values of Il-18 and Epo were found in BB.6S. Il-18 is the only cytokine with a unique capacity to induce T helper 1 (Th1) or Th2 polarization, depending on the immunologic context,13 and Epo exerts cytoprotective effects.14 In our study, it seems that both Il-18 and Epo have a diabetes-protective effect in BB.6S rats, because only 10% of them develop T1D despite being homozygous for the essential diabetogenic genes Iddm1 and Iddm2. Ccl3 showed the highest diabetes-protective effect, which occurred in all BB.6S rats at a similarly high level, whereas Ccl3 in the other strains was detectable in the plasma of only a few (BB/OK, BB.4S) or no rats (BB.1K). This can be supported by the fact that Ccl3 in knockout mice which do not produce Ccl3 showed reduced destructive insulitis and were protected from diabetes.15
The results of this study indicate that BB.6S rats must have completely different mechanisms leading to diabetes protection than are present in BB.4S or BB.1K. If this finding is true, BB/OK should be protected by the Ccl3 deficiency observed here, but they develop up to 100% T1D up to an age of 30 weeks. Furthermore, histological studies of BB.6S rats have shown that more than 50% of islets were infiltrated at an age of about 30days, whereas BB/OK rats showed an infiltration rate of only about 5%.9 This may mean that the islet infiltration in BB.6S is a benign form which should now be supported by high IL-18 and Epo concentration in plasma at 4weeks of age.
We thank Silvia Sadewasser for expert technical assistance. This research is partially supported by Else Kroner-Fresenius-Stiftung 2011_A62.
The authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.