Journal of
eISSN: 2374-6947


Research Article Volume 1 Issue 1
1Department of Endocrinology and Metabolism, Federal University of Sao Paulo (UNIFESP), Brazil
2Department of Endocrinology and Metabolism, Federal University of Goias (UFG), Brazil
3Nove de Julho University (UNINOVE), Brazil
4Department of Pathology, Federal University of Sao Paulo (UNIFESP), Brazil
5Department of Biophysics, Federal University of Sao Paulo (UNIFESP), Brazil
Correspondence: Claudio E Kater, Department of Endocrinology and Metabolism, Federal University of Sao Paulo, Rua Pedro de Toledo, 781 – 13o, andar 04039-032 Sao Paulo, SP – SA, Brazil, Tel +551155746502
Received: May 24, 2014 | Published: June 4, 2014
Citation: Kater CE, Antunes DE, Silva JA, et al. 11 β-hydroxysteroid dehydrogenase type 1 is not over expressed in cushing’s syndrome adipose depots. J Diabetes Metab Disord Control 2014;1(1):21-25. DOI: 10.15406/jdmdc.2014.01.00006
Glucocorticoids have a major role in adipose tissue distribution, partially regulated at a pre-receptor level by 11b-hydroxysteroid dehydrogenase type 1 (11b-HSD1). 11b-HSD1 generates intracellular cortisol promoting adipocyte differentiation. The distinctive expression of 11bHSD1 and glucocorticoid receptor-a (GRa) may explain the preferential abdominal fat depots in Cushing’s syndrome (CS).
Aim: To quantify and evaluate the effects of chronic exposure to hypercortisolism on the expression of 11b-HSD1 and GRa in adipose depots of patients with CS.
Subjects and Methods: Samples of visceral (VAT) and subcutaneous adipose tissue (SAT) were obtained during elective abdominal surgery from female patients with CS (n=10), obese (n=15) and nonobese controls (n=10), in whom body mass index (BMI), abdominal circumference (AC) and salivary cortisol (SF) were previously determined. 11b-HSD1 and GRa expressions were quantified by real-time PCR.
Results: 11b-HSD1 expressions in SAT and VAT of CS were not different from nonobese and were upregulated in obese (P<0.0001 and P<0.05, respectively). Additionally, GRa mRNA was downregulated in SAT of obese and CS (P<0.0001 for both). In the whole group, neither 11b-HSD1 nor GRa levels showed correlation with SF. However, 11b-HSD1 mRNA correlated positively with BMI and AC in SAT and VAT and GRa correlated negatively with both in SAT.
Conclusions: Chronic hypercortisolism, as seen in CS, does not result in upregulation of 11b-HSD1 expression in adipose depots, in contrast with In vitro observations. This may suggest a protective mechanism, since 11b-HSD1 is emerging as a key component in homeostatic adaptation, rather than the cause of visceral obesity.
Key words: visceral obesity, cushing’s syndrome, 11β-HSD1, GRα
AC, abdominal circunference; 11β-HSD1, 11b-hydroxysteroid dehydrogenase type 1; BMI, body mass index; CS, cushing’s syndrome; GRα, glucocorticoid receptor-a; SF, salivary cortisol; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; GC, glucocorticoids
Glucocorticoids (GC) have a major role in determining adipose tissue distribution and metabolism. Subjects with endogenous or exogenous hypercortisolism develop a central obesity pattern that is reversible upon treatment or GC withdrawal.1 The mechanisms involved in GC-mediated adipose tissue distribution are not completely understood. Part of GC action is regulated at a pre-receptor level by 11b-hydroxysteroid dehydrogenase type 1(11b-HSD1), an NADPH-dependent enzyme highly expressed in the liver and adipose tissue, where it is co-localized with the GC receptor-a (GRa). In most intact cells and tissues, 11b-HSD1 functions as a reductase, converting inactive cortisone to active cortisol.2 GC regulates multiple processes in the adipose tissue:
Transgenic mice overexpressing 11b-HSD1 in adipose tissue develop obesity with all features of the metabolic syndrome,6 whereas 11b-HSD1-knockout mice are protected from both.7 The bulk of evidences points to an overexpression and increased activity of 11b-HSD1 also in human adipose tissue,8‒11 although there are contrasting data.12 Serum cortisol levels are not elevated in obesity;13instead, it may be locally increased in the adipose tissue due to a greater activity of 11b-HSD1.
There are striking similarities between Cushing’s and the metabolic syndrome. Besides, omental adipose cells cultured with cortisol or dexamethasone plus insulin showed increased expression5 and activity5,14 of 11b-HSD1. Moreover, enlarged abdominal fat cells are seen in Cushing’s syndrome (CS) and 11b-HSD1 activity is correlated to adipocyte size.14 All these observations led to the speculation that 11b-HSD1 gene expression is upregulated in the Visceral Adipose Tissue (VAT) of subjects with CS. However, Mariniello et al.,15 showed recently no differences in omental 11β-HSD1 mRNA expression between Cushing´s syndrome and non-obese controls.
Relying on the evidences that 11b-HSD1 mRNA expression is closely related to 11b-HSD1 activity,9 we examined the hypothesis that 11b-HSD1 and GRa are distinctively expressed in subcutaneous and visceral compartments of CS and obese patients.
Subjects/procedures
The study, previously approved by the local Ethics Committee, encompassed 10 female patients with adrenal CS and 25 female control subjects who gave their full, informed, written consent.
Body Mass Index (BMI), Abdominal Circunference (AC) and hip circumferences were determined. The control group was stratified according to BMI, into two subgroups: the nonobese (BMI<30Kg/m2) with 10 subjects, and the obese with 15, including 7 class I/II (BMI³30 and <40Kg/m2) and 8 class III (BMI³40Kg/m2). Exclusion criteria included the presence of any inflammatory and/or malignant condition, diabetes, fasting impaired glucose (>5.6 mmol/liter) or current use of medications known to interfere with 11β-HSD1 expression or function, such as steroids, metformin, glitazones and anorexigens.
The diagnosis of adrenal CS was established before surgery by elevated 23:00h salivary cortisol (SF), lack of cortisol suppression after overnight 1mg oral dexamethasone, increased 24h urinary free cortisol excretion and undetectable plasma ACTH levels and confirmed in all on pathology grounds.
Approximately 2g subcutaneous and visceral adipose tissue samples were obtained from the patients with CS during videolaparoscopic adrenalectomy for a cortisol-secreting adrenal tumor and from control subjects during elective abdominal surgery (colecistectomy in 13, bariatric surgery in 10, ovarian cystectomy in one and tubal sterilization in one). Hydrocortisone replacement therapy was started in CS patients after biopsies were collected. Samples were immediately frozen in dry ice and stored at -70°C until RNA extraction.
The night before surgery, all patients remained fast after 22:00h and were instructed to collect saliva at 23:00h in a specific collector (SalivetteÒ, Sarstedt, Germany).
Measurement of salivary cortisol
Saliva samples were centrifuged at 2,000 rpm and kept frozen until assay. Salivary cortisol was measured in 25µl saliva aliquots by an in-house radioimmunoassay (RIA), as previously described.16 In brief, the intra- and inter-assay coefficients of variation were 4.4% and 5.1% respectively, with a detection limit of 10ng/dL.
Total RNA was isolated from SAT and VAT by TRIzol reagent (Gibco BRL, Gaithersburg, MD, USA), according to the manufacturer’s protocol. RNA was subjected to DNAse I digestion and quantified using spectrophotometric analyses (ND-1000, NanoDrop, Wilmington, DE, USA). RNA integrity was assessed by electrophoresis on 1% agarose gel. Oligo-(deoxythymidine)-primed cDNA was synthesized from 5mg of total RNA using M-MLV (Moloney murine leukemia virus) protocol (Invitrogen). A standard curve for each pair of primers was generated by serial dilution of cDNA randomly selected from six subcutaneous and six visceral samples.
Real-time PCR
PCR was performed in a 7000 Sequence Detection System (ABI Prism, Applied Biosystems, Foster City, CA, USA) using the SYBRGreen core reaction kit (Applied Biosystems). Primers used for 11b-HSD1, GRa, PPARg1, and PPARg2 mRNA quantifications were as follows:
11b-HSD1, 5´-GCAGCCTCAGCACACTACATTG-3´ (forward),
5´-GGTGATGTGGTTGAGAATGAGC-3´ (reverse)
(GenBankTM accession number J00691);
GRa, 5´-CCCCAGGTAAAGAGACGAATG-3´ (forward),
5´-CGGTAAAATGAGAGGCTTGCA-3´ (reverse)
(GenBankTM accession number NM_030851);
All reactions were multiplexed with the housekeeping human 18S rRNA gene with the following sequence: 5´-GTAACCCGTTGAACCCCATT-3´ (forward),
5´-CCATCCAATCGGTAGTAGCG-3´ (reverse)
All results were confirmed using the housekeeping ARPO gene (data not shown).
Each sample was run in duplicate and the mean of duplicate was used to calculate transcript level.
Quantitative values for 11b-HSD1, GRa and 18S rRNA mRNA transcription were obtained from the threshold cycle (Ct) number, where the increase in the signal associated with an exponential growth of PCR products begins to be detected. Melting curves were generated at the end of every run to ensure product uniformity.
The relative target gene expression level was normalized on the basis of 18S rRNA expression as endogenous RNA control. ΔCt values of the samples were determined by subtracting the average Ct value of 11b-HSD1 and GRa mRNA from the average Ct value of the internal control 18S gene. Reactions were performed as follows: 50°C for 2 min, 95°C for 10min and then 50 cycles of 95°C for 15sec and 60°C for 30s and a dissociation cycle.
Statistical analysis
Statistical analysis was performed using the SPSS software 16.0.1 version for Windows (SPSS Inc. Chicago, IL, USA). Comparisons between groups were performed by ANOVA for variables with a normal distribution and Kruskal-Wallis for non-parametric variables, whereas Pearson and Spearman tests were used to verify correlation between variables. Multiple regression analyses were employed to adjust for the influence of BMI and AC. The data are expressed as mean±SD, unless otherwise stated. Differences were considered statistically significant when P was less than 5%.
Subjects characteristics
Clinical and biochemical characteristics of patients with CS, nonobese and obese subjects are shown in (Table 1). BMI of Cushing’s patients were similar to nonobese (28.5±4.0 vs 25.5±2.2Kg/m2) and significantly lower than obese class I/II and class III (33.5±3.7Kg/m2; P<0.05 and 46.3±4.3Kg/m2; P<0.0001 respectively). Abdominal and hip circumferences were significantly larger in obese class III than in Cushing’s patients (125±15.2 vs 97.8±17.7cm; P<0.001 and 136.7±17.6 vs 103.9±11.8cm; P<0.0001 respectively), nonobese (P<0.0001 for both) and obese I/II (P=0.05 and P=0.001 respectively). Similar to BMI, abdominal and hip circumferences in CS were closer to those in nonobese.
Cushing’s syndrome (n=10) |
Nonobese |
Obese class I/II (n=7) |
Obese class III (n=8) |
|
Age(years) |
42.1±17.7 |
41.5 ±17 |
58.6±14.5 |
41.9±12 |
[21–81] |
[20–80] |
[42–79] |
[27–57] |
|
BMI(Kg/m2) |
28.5±4.0**,*** |
25.5±2.2 |
33.5±3.7† |
46.3±4.3††,£ |
[22.6–35.3] |
[21.3-28.3] |
[30–39] |
[40.4–53] |
|
Abdominal circumference(cm) |
97.8±17.7*** |
87.9±7.3 |
104.8±10.3 |
125±15.2††,£ |
[96–120] |
[78–100] |
[90-120] |
[105‒153] |
|
Hip circumference(cm) |
103.9±11.8*** |
96.5±4.3 |
110.5±7.8 |
136.7±17.6††,£ |
[84–130] |
[9–103] |
[102–120] |
[112–158] |
|
23:00h Salivary cortisol (ng/dL) |
1,114±805*,**,*** |
182±105 |
126.6±70 |
108.5±40 |
[293–2,680] |
[57–347] |
[22–235] |
[53–152] |
Table 1 Clinical and biochemical characteristics of the female patients studied
All data are mean±SD, followed by range in brackets.
P<0.05 *Cushing’s syndrome (CS) vs nonobese; **CS vs obese class I/II; ***CS vs obese class III; †Obese class I/II vs nonobese; ††Obese class III vs nonobese Obese class III vs obese class I/II
Salivary cortisol at 23:00h was noticeably higher in Cushing’s (30.7±22.2nmol/L) than in nonobese (4.9±2.8nmol/L; P<0.0001), obese class I/II (3.5±1.9nmol/L; P<0.0001) and class III (3±1.1nmol/L; P<0.001), but did not differ among the latter 3 groups. Means (±SD) of age, BMI, AC and SF in the whole obese group were: 49.6±15.4years; 40.3±7.6Kg/m2; 115.6±16.4cm and 3.2±1.6nmol/L respectively.
Comparison of 11β-HSD1 and GRa mRNA levels in subcutaneous and visceral adipose tissue
In CS, 11b-HSD1 expressions in SAT and VAT were not statistically different from those in nonobese (0.35±0.1 vs 0.17±0.06 and 1.3±0.56 vs 0.95±0.36 respectively) (Figure 1), whereas they were significantly lower than in obese only in SAT (0.35±0.1 vs 0.64±0.3; P=0.01). In obese, 11b-HSD1 mRNA levels were higher than in nonobese patients both in SAT and VAT (0.64±0.3 vs 0.17±0.06; P<0.0001 and 1.6±0.7 vs 0.95±0.36; P<0.05 respectively).
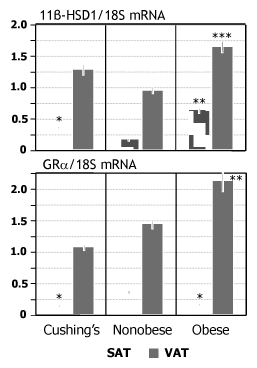
GRa mRNA expressions in SAT of Cushing’s and obese subjects were significantly lower than in nonobese (0.14±0.08 and 0.17±0.09 vs 0.36±0.1; P<0.0001 for both) (Figure 1). However, in VAT, GRa mRNA expression was higher in obese class I/II than in Cushing’s patients (2.81±1.6 vs 1.07±0.26; P<0.05).
A significant correlation between visceral, but not subcutaneous, 11b-HSD1 and GRa mRNA levels was observed when the analyses was performed either with the whole group (nonobese, obese and Cushing’s, r=0.6; P<0.0001) or with CS and obese subjects individually (r=0.87; P=0.001 and r=0.74; P<0.005, respectively) (Figure 2).

Association of 11β-HSD1 and GRa with anthropometric and biochemical parameters
In CS patients, 11b-HSD1 expression in VAT, but not in SAT, correlated positively with AC (r=0.66; P<0.05 and r=0.68; P<0.05 respectively). In contrast, GRa expression did not correlate with BMI or AC in CS. In the whole group, 11b-HSD1 expressions in SAT and VAT were positively and significantly correlated with BMI (r=0.43; P=0.01 and r=0.35; P<0.05 respectively) and with AC (r=0.36; P<0.05 and r=0.4; P<0.01 respectively). Additionally, GRa mRNA levels in SAT, but not in VAT, were negatively correlated with BMI and AC in the whole group (r=-0.37; P<0.05 and r=-0.45; P<0.01 respectively) (Table 2).Salivary cortisol did not correlate with 11b-HSD1 or GRa mRNA expressions in VAT or SAT of CS. When 11b-HSD1 and GRa were analyzed in the whole group (by stepwise multiple regression), the former was the best predictor of BMI (P=0.01) and the latter the best predictor of AC (p<0.01) both in SAT.
|
11b-HSD1 |
G Ra |
||
|
SAT CS |
VAT CS |
SAT CS |
VAT CS |
|---|---|---|---|---|
BMI(Kg/m2 ) |
NS |
NS |
NS |
NS |
AC(cm) |
NS |
r= +0.6 |
NS |
NS |
P<0.05 |
||||
SAT WG |
VAT WG |
SAT WG |
VAT WG |
|
BMI (Kg/m2 ) |
r=+0.43 |
r=+0.35 |
r=-0.37 |
NS |
P= 0.01 |
P<0.05 |
P<0.05 |
||
AC(cm) |
r=+0.36 |
r=+0.40 |
r=-0.45 |
NS |
P< 0.05 |
P<0.01 |
P<0.01 |
|
|
Table 2 Correlation of 11b-HSD1 and GRa levels with anthropometric parameters in the whole group (WG) and Cushing’s patients (CS)
BMI, body mass index; AC, abdominal circumference
In the present study, we found no significant differences between 11b-HSD1 mRNA expressions in SAT and VAT from Cushing’s patients as compared to nonobese controls, although they were greater in CS. The only published study on CS analyzed 11b-HSD1 mRNA expressions in VAT and it is in accordance with our findings. Furthermore, 11b-HSD1 expression in SAT was greater in obese controls than in Cushing’s patients. At present, there are no data available regarding 11b-HSD1 mRNA expressions in SAT of CS subjects. Of interest, both ours and Mariniello’s study15 are not in accordance with a previous in vitro experiment, which demonstrated increased activity and expression of 11b-HSD1 in human omental adipose cells cultured with cortisol and insulin, suggesting that obesity could be “Cushing’s disease of the omentum”.5 Thus, it is anticipated that both systemic hypercortisolism and cortisol generated from 11b-HSD1 in an autocrine manner, could promote adipocyte differentiation4 and proliferation,17 as seen in stromal cells in vitro. However, Lee et al.14 recently found decreased 11b-HSD1 mRNA expression in VAT cultured with dexamethasone alone, corroborating our findings. Furthermore, mRNA levels were decreased when insulin was added to dexamethasone in the culture, compared to insulin alone, whereas reductase activity paradoxically increased.
Despite similarities between Cushing’s and the metabolic syndrome, additional implications from in vitro study and the enlarged abdominal fat cells seen in CS, chronic exposure to cortisol in vivo seems not to upregulate 11b-HSD1 expression in both SAT and VAT. Moreover, whole adipose tissue may have a different response to hypercortisolism when compared to adipose stromal cells. Although 11b-HSD1 expression is remarkably correlated with its activity,9 further studies on 11b-HSD1 activity and the apparent synergic action of cortisol and insulin are needed in the CS.
On the other hand, we demonstrate that 11b-HSD1 mRNA expression is upregulated in both SAT and VAT of obese subjects, in agreement with preliminary studies performed in SAT.8‒11 Although VAT appears biologically more active than SAT, there is only a few controversial studies in VAT: one observed increased 11b-HSD1 expression18 and others did not.12,19 Moreover, 11b-HSD1 mRNA expression was positively correlated with BMI and AC both in SAT and VAT, as shown in other studies.9‒11,15 However, 11b-HSD1 mRNA expression did not correlate with systemic cortisol in CS patients or in other groups, suggesting that 11b-HSD expression in adipose tissue is not dependent on systemic cortisol levels, but is really dependent on BMI. It is important to note that BMI was not different in controls and CS.
GRa mRNA expression in VAT also showed a strong and positive correlation with 11b-HSD1 in the whole group and in obese and Cushing’s groups separately, strengthen the theory that co-expression of these two genes may amplify glucocorticoid action locally.2 Additionally, the inverse correlation of GRa mRNA expression with BMI and AC in SAT is in agreement with Zoi et al.18 Besides, the decreased GRa mRNA expression in SAT observed in obese patients may reflect a compensatory downregulation to increased 11b-HSD1, as suggested by Boullu-Ciocca et al.20 On the other hand, GRa mRNA expression in VAT of Cushing’s and obese subjects did not undergo downregulation, despite the 11b-HSD1 increment in obese. The absence of this protective mechanism in VAT could contribute to obesity related metabolic complications. As for CS, this is the first report to evaluate GRa expression in adipose tissue. Chronic hypercortisolism in vivo seems to result in downregulation of GRa gene expression in both SAT and VAT. Indeed, health volunteers treated for one week with prednisolone had a 50% decrease both in GR protein and mRNA levels in subcutaneous abdominal biopsies.21
It has been recognized that large abdominal adipose depots are closely linked to cardiovascular complications. However, some evidences suggest that 11b-HSD1 may not hold a good relationship with body composition in VAT.12,19 Indeed, we found that the best predictors of BMI and AC in SAT, but not in VAT, were respectively 11b-HSD1 and GRa. Biopsying subcutaneous depots is a much easier procedure, so that these observations will facilitate methodology of future studies.
11b-HSD1 is now emerging as a key component in homeostatic adaptation, rather than the cause of visceral obesity or metabolic syndrome. Recent studies suggest that the enzyme is influenced by the nutritional status. Accordingly, the lack of 11b-HSD1 increase in CS may suggest a protective mechanism against the metabolic complications. Indeed, when the opposite occurs, e.g. weight loss in simple obesity, 11b-HSD1 undergoes upregulation,22 although this is not a universal finding.11 Thus, there are several evidences suggesting that 11b-HSD1 adjusts local cortisol concentration independently of its circulating levels.
In summary, 11b-HSD1 gene expression is up-regulated in obesity, but not in CS. In addition, GRa mRNA level is downregulated in SAT of Cushing’s patients. The expected upregulation of 11b-HSD1 gene expressions in VAT of Cushing’s patients was not observed.
None.
Author declares that there is no conflict of interest.

©2014 Kater, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.