Journal of
eISSN: 2574-9943


Research Article Volume 7 Issue 4
1MD (Skin) Medical Investigator, MBBS (Principal Investigator), PhD Research Scholar, Director and Head, NovoBliss Research Private Limited, Ahmedabad, India
2Anveya Living Private Limited
Correspondence: Maheshvari Patel, Address NovoBliss Research Pvt. Ltd., Office# A - 206, 2nd Floor, Shaligram Lakeview, Shopping Complex, No.Vaishnodevi Circle, Khoraj, Gandhinagar – 382421, Gujarat, India, Tel +91-79- 4898 3895
Received: October 01, 2023 | Published: October 16, 2023
Citation: Merja A, Nayan P, Maheshvari P, et al. Safety and efficacy of Arcedin™ infused anti-gray hair serum in adult human subjects with partially gray hair. J Dermat Cosmetol. 2023;7(4):115-123. DOI: 10.15406/jdc.2023.07.00247
Background: Hair graying is a common sign of aging resulting from complex regulation of melanogenesis. It occurs due to many factors, including age, oxidative stress, psychological stress, and malnutrition.
Objective: Assessment of safety and effectiveness of an anti-gray hair product when applied to healthy adult human subjects having partially gray hair.
Methods: Total 32 subjects, aged 20 to 55 years, were initially enrolled and 29 subjects successfully completed the study, which spanned a total of five visits over 120 days. The subjects were provided with ThriveCo® Hair Prime Serum as test product and adjunct shampoo to use at home. Hair serum was evenly distributed across scalp and gently massaged into the hair roots. Primary evaluations included reduction in hair graying, improvement in hair growth, and Graying Severity Score (GSS) for reduction in graying severity.
Results: After 120 days of treatment, the GSS exhibited a significantly decrease (p-value <0.01) to 7.00, indicating significant effect of the hair serum in reducing severity of hair graying. Additionally, a substantial improvement (p-value <0.01) in hair growth was observed, with 86.57% and 13.41% of hairs in the anagen (A) stage and telogen (T) stage respectively (A:T ratio= 6:1). Image-Pro analysis using the L* value demonstrated marked improvement of 6.19% on Day 120 from baseline Day 01, further indicating the anti-graying effect of the hair serum. Furthermore, all the subjects experienced visible enhancement in general appearance of hair and scalp. None of the subjects experienced any adverse events during entire study duration.
Conclusion: Test product ThriveCo® Hair Prime Serum containing Arcedin™ demonstrated efficacy and tolerability in reversing the hair graying. Furthermore, following a 120-day usage, this hair serum demonstrated significant enhancements in hair growth, visual appearance, and strength, solidifying its potential as a beneficial addition to a comprehensive daily hair care regimen.
Keywords: hair density, phototrichogram, graying severity score, hair thickness, pluck test, trichogram
GCP, good clinical practice; CTRI, clinical trial registry of India; IUD, intrauterine device; GSS, graying severity score
The graying of hair is among the initial and most conspicuous signs of aging in humans. This phenomenon holds considerable social significance across diverse cultures, geographic regions, and ethnic backgrounds. Simultaneously, there is a growing global interest in finding ways to reverse this process.1
Hair pigmentation is one of the most unique features in humans ranging from black, brown, and blonde to red. The color of human hair is due to pigment melanin produced by melanocytes which are neural crest derivatives. Human hair follicles contain two types of melanin as follows: eumelanin and pheomelanin. The diversity of hair color arises mostly from the quantity and ratio of black-brown eumelanin and reddish-brown pheomelanin. It has been hypothesized that the pH and cysteine level of melanosomes influences the phenotype of hair. As pH reduces, there is a progressive reduction in tyrosinase activity leading to increased pheomelanin and reddish or blonde hair. There are various differences between pigmentation in the skin and that of hair. Each melanocyte is associated with five keratinocytes in the hair bulb forming a “hair follicle-melanin unit.” In contrast, each melanocyte in the skin is associated with 36 keratinocytes constituting an “epidermal-melanin unit.” Unlike in the skin where pigment production is continuous, melanogenesis in the hair is closely associated with stages of the hair cycle. Hair is actively pigmented in the anagen phase and is “turned off” during the catagen phase and absent during telogen.2
The pigmentary unit is a pear-shaped black structure at the tip of dermal papilla in pigmented hair.3 The white of canities is because of an optical illusion. The pale yellow of keratin appears white due to reflection or refraction of incident light.4 Gray hair has some color with sparsely distributed melanosomes; however, white hair is completely deprived of melanosomes and color. White hair occurs only on the scalp.5
The test product used in this study is made up of Arcedin™ which is a mixture of Aqua and Propanediol, Citrus Reticulata Extract/Citrus Reticulata (Tangerine) Extract and Acetyl Tyrosine and Pentylene Glycol and Gluconolactone and Sodium Benzoate and Aqua/Water and Biotin. Arcolys is a natural active ingredient present in the test product which is derived from the molecule Picroside II which reverses graying of hair and restores the natural hair color. Melanogray is an anti-hair graying essence that is sustainably obtained by upcycling the peel paste from unique organic Chios mandarins.6 It also contains Biotin, also known as vitamin H or B7 which helps in the metabolism of fats, carbohydrates, and protein. Biotin helps in maintaining normal skin, normal hair, normal macronutrient metabolism, and normal immune system function. Premature graying is an important cause of low self-esteem, often interfering with socio-cultural adjustment.7
This clinical study was done to assess the safety and effectiveness of Anti-Gray Hair Product in Healthy Adult Human Subjects with Partially Gray Hair. The study was done to demonstrate the continuous use of the ThriveCo® Hair Prime Serum for 120 Days with the aim to reverse the gray hair, improve hair condition, scalp condition, and hair strength.
Ethical conduct of the study
The study was conducted according to the declaration of Helsinki (Brazil, October 2013), Good Clinical Practices (GCP) for clinical research in India 2005, new drugs and clinical trials rules 2019, ICH GCP E6 (R2) guidance on good clinical practice, and with ICMR's National ethical guidelines for biomedical and health research involving human participants, 2017. Independent ethics committee had approved the study protocol [version#01 (Final)], informed consent form [version#01 (Final)], case report form [version#01 (Final)] and other necessary documents before commencement of the study procedures. This clinical study was registered with the Clinical Trial Registry of India (CTRI) with CTRI# CTRI/2023/01/04886.
Overall study design and plan
This was a single-arm, single-center, clinical study designed to evaluate the safety and efficacy of an anti-gray hair product among a group of healthy adult human subjects having partial graying of hair. Total 32 subjects, aged 20 to 55 years were enrolled in the study. Prior to their participation, a written informed consent was obtained from each subject. The study had duration of 120 days, commencing on the day of enrolment, and included a sequence of five visits scheduled at Day 01, Day 30, Day 60, Day 90, and Day 120. Subjects underwent screening based on predetermined inclusion and exclusion criteria. Throughout the study, a comprehensive set of different parameters were evaluated at various time points to assess the efficacy and safety of the hair serum. Efficacy assessments encompassed quantifying gray and non-gray hair using CASLite-Nova (Catseye Systems & Solutions Pvt Ltd, India), followed by analysis using Image-Pro software (Version 10.0.13) (Media Cybernetics Inc., Unites States) to determine the ratio of non-gray to gray hair. Further evaluations included an improvement in the Anagen to Telogen (A:T) ratio by employing the hair pluck test, degree of hair graying using the Graying Severity Score (GSS), assessment of the product's impact on hair growth and hair morphology, and an assessment of general appearance of hair and scalp. General appearance of hair was evaluated considering improvements in hair parameters including hair volume, hair density, hair plasticity, hair reflection and smoothness. The general scalp appearance was also assessed, focusing on parameters such as itchiness, redness, roughness, and scaliness, alongside hair strength. Subjects' perception of the product was gauged using the Subject Response Index, capturing their overall feedback and satisfaction as consumers. Safety assessment comprised of inquiries and evaluations regarding any treatment-emergent adverse events, including scalp redness, dryness, and unbearable irritation, tingling, and burning sensations. These assessments were conducted by the Dermatologist and its trained evaluator.
Dermatologist-trained evaluator was a study personnel with relevant experience in conducting research and had qualification as a paramedic, who was trained by dermatologist for the evaluation of subjects using different assessment methods which are used in this clinical study.8
Study participants and criteria
32 healthy adult subjects aged 20 to 55 years were enrolled with the aim of obtaining 30 evaluable healthy adult subjects at the conclusion of the study. Ultimately, 29 subjects successfully completed the study.
Inclusion criteria
The study enrolled healthy subjects aged 20 to 55 years of both the genders who had partial graying of hair determined by medical examination and thorough evaluation of history conducted by the investigator. In order to be eligible for participation, individuals had to willingly provide written informed consent and commit to completing all study-related activities, including regular follow-up appointments. Female participants who remained on a stable regimen of contraceptive or replacement hormonal therapy or those who were not undergoing any such therapy, for at least 6 weeks before the study and throughout its duration were included in the study. Additionally, female participants of childbearing potential were required to commit to maintaining an established method of birth control, which could include options such as an Intrauterine Device (IUD), hormonal implant device/injection, regular use of birth control pills or patches, diaphragms, condoms with spermicide, sponges with spermicidal jelly, cream, or foam, partner vasectomy, or practicing abstinence. Furthermore, female participants who were not of childbearing potential due to surgical sterility, having been post-menopausal for at least 1 year, or having undergone a tubal ligation were also considered for inclusion. These individuals were required to pledge not to use medicated or prescription shampoos, hair care products containing Minoxidil, or any other hair growth or hair care products apart from the test product for the entire duration of the study. Subjects who consistently adhered to these criteria and agreed to use the test product throughout the entire study period were included in the research.
Exclusion criteria
In this clinical study, stringent exclusion criteria were applied to ensure the selection of highly specific subjects. Excluded were individuals with non-gray hair color, a documented history of scalp dermatological conditions other than hair loss or dandruff, a history of alcohol or drug addiction, chronic illnesses potentially affecting the skin, prior use of hair growth treatments within 3 months, or any previous hair growth procedures (e.g., hair transplant or laser therapy). Subjects who had undergone topical treatments for a minimum of 4 weeks or systemic treatments for at least 3 months prior to study entry and those using other commercial hair fall control or hair growth products during the study along with usage of chronic oral steroids 3 months prior to and during the study were ineligible for participation in the study. Additionally, individuals with irritated or inflamed scalps, severe scalp diseases with allergic responses to cosmetic products, pregnant or breastfeeding females, or those planning pregnancy during the study period were excluded. Further exclusions comprised subjects involved in similar cosmetics, devices, or therapeutic trials within the past four weeks, individuals considered unsuitable for enrolment by the investigator or expert physician, and those unwilling to provide informed consent.
Test product
The test product, ThriveCo® Hair Prime Serum is an anti-gray hair serum which is researched and developed by Anveya Living Private Limited. It is a blend of active ingredient like Arcedin™ which is a mixture of Aqua and Propanediol, Citrus Reticulata Extract/Citrus Reticulata (Tangerine) Extract and Acetyl Tyrosine and Pentylene Glycol and Gluconolactone and Sodium Benzoate and Aqua/Water and Biotin. Other active ingredients of test product include Aloe barbadensis Extract, Polyquaternium-7, Camellia Sinensis Leaf Extract, Panthenol, and Rosmarinus officinalis. Its inactive ingredients include, Propylene Glycol, Propanediol, Xylitylglucoside, Anhyroxylitol, Xylitol, Phenoxyethanol, Hydroxypropyl Methylcellulose, Sodium Benzoate and Potassium Sorbate. In addition to the test product, subjects were provided with ThriveCo® Hair Vitalizing Shampoo as an adjunct product to use it along with the test product in order to standardize the shampoo used during study period (Table 1).
|
Parameter(s) |
Treatment |
|
Product Name |
ThriveCo® Hair Prime Serum Active ingredients: Arcedin™ [Aqua (and) Propanediol, Citrus Reticulata Extract/Citrus Reticulata (Tangerine) Extract (and) Acetyl Tyrosine (and) Pentylene Glycol (and) Gluconolactone (and) Sodium Benzoate (and) Aqua / Water and Biotin], Aloe barbadensis Extract, Polyquaternium-7, Camellia Sinensis Leaf Extract, Panthenol, Rosmarinus officinalis Inactive ingredients: Propylene Glycol, Propanediol, Xylitylglucoside, Anhyroxylitol, Xylitol, Phenoxyethanol, Hydroxypropyl Methylcellulose, Sodium Benzoate, Potassium Sorbate. ThriveCo® Hair Vitalizing Shampoo (Adjunct Product) |
|
Storage condition |
The test product was stored in cool and dry place below 25 ° C. |
|
Dosage Form |
Hair Serum |
|
Dosage |
Use the dropper (1 mL) to dispense the product evenly on the scalp and gently massage on the roots of the hair. |
|
Route of Administration |
Topical |
|
Marketed by |
Anveya Living Private Limited |
Table 1 Test Product
All the enrolled subjects were instructed to take full dropper (1 mL) of test product and gently massage this no-rinse formula on the roots of the hair. The test product was applied at night before going to bed consecutively for 120 days.
Dermatologist evaluation
The changes in general appearance of hair and scalp were evaluated by dermatologist trained evaluator under supervision of dermatologist on Day 01, Day 30, Day 60 and Day 120 as compared to baseline visit. Changes in general appearance of hair was assessed based on standard clinical questionnaire with responses being categorised accordingly. The questionnaire included evaluation for hair volume, meaning the amount of scalp hairs, by categorizing it into full, medium or small. Similarly, hair reflection (measure of how hair appears when light was projected on it) was categorised into shiny or blunt, hair density (measure of number of hair strands growing at each square-inch of the scalp) into dense or thinned/shade, hair plasticity (ability of hair to be reshaped or moulded, into waved or flat, hair shininess (measure of how shiny the hairs appear) into poor, average or good and hair smoothness (measure of how smooth and frizz free your hairs are) into poor, average or good. Subsequently, general appearance of scalp was evaluated in terms of experiencing concerns like scalp itchiness, dryness, redness, roughness and scaliness.9
Subjective product perception assessment was done based on a 5-point scale, with 1 meaning no experience at all and 5 meaning experiencing to a large extent.
Phototrichogram
Phototrichogram stands out as the best non-invasive and reproductible method for quantification of hair growth parameters9. Phototrichogram was done for all the enrolled subjects by taking pictures with CASLite-Nova to obtain number of white/gray hairs, non-white/gray hair, and a ratio of non-gray to gray hair calculated based on Image-Pro software analysis. A total of five tattoos were made covering each, frontal region, vertex region, right and left temporal regions, and the occipital region. Standardization of location was done by a tattoo made by permanent ink marker in order to get reading from the same site during each successive visit. In order to confirm tattoo area, a digital photograph of the head crown was taken.
Study staff selected a small area of 1x1 cm2 on the subject’s scalp, 15 cm from the tip of the nose and marked as frontal area. Similarly, marking of vertex region was done as a scalp region 30 cm from the tip of the nose. 5 cm mark below the vertex region was considered as occipital region. To define the right and left temporal regions, markings were made at points 6 cm above the apex of each ear. The pictures were taken by CASLite-Nova at each visit and from the same site to count ratio of non-gray hair to gray hair considering it as baseline reading. Post that analysis of the captured images was done using Image-pro software.
Graying severity score9
Based on the hair count from the image captured during phototrichogram by CASLite-Nova at each visit and from the same site, a score was assigned to all 5 scalp zones based on the percentage of gray hair in that zone. The scores were calculated as follows: Score 1 (for less than 10% gray hair/cm2); Score 2 (10% - 30% gray hair/cm2); and Score 3 (more than 30% gray hair/cm2). Finally, the GSS was calculated for each patient by summing up the scores at all five standardized sites with the maximum achievable score being 15 (3 × 5). Obtained scores were further graded as Mild (0–5); Moderate (6–10) and Severe (11–15).
Hair pluck test9
For performing this test, hairs were taken from the vertex region (marked 30 cm from the tip of nose) from the predefined site on the day of enrolment. Surrounding hairs were fixed with clips, and approx. 10 to 30 hairs were firmly grasped and tightly plucked using forceps/rubber-protected jaws ensuring the plucking occurred as close to the scalp as possible. Care was taken to avoid dystrophic and broken hairs, while also targeting the extraction of miniaturized hairs when present. Number of anagens and telogen hair were recorded and the Anagen to Telogen (A:T) ratio was calculated. Images and slides were preserved for reference. Similarly, post baseline pluck test was performed at site 2 cm adjacent to site used at baseline. Standardization of the site was done for all the subjects throughout the study to obtain consistent reading and subsequent results.
Hair pull test10
It was a semi-quantitative clinical impression about how easy the hair falls from scalp. During this test, around 60 hair shafts were held between the thumb, middle and index finger, holding close to scalp surface and pulled firmly away from the scalp along with hair shaft up to the upper hair tip. Counting of pulled hair was than done.
Statistics
Categorical variables were expressed by frequency and percentage along with the graphical presentation whenever required. For continuous variables, data were compared from baseline to post-treatment using Paired t-test. The statistical analysis was done by using SPSS software (Version: 26.0) with 95% confidence interval and 5% level of significance. Withdrawn subjects were not included in the statistical analysis.
GSS is a measure of degree of hair graying. Based on the evaluation done the average GSS of subjects at Day 1 was 13.03 (categorized as severe) which was decreased throughout the successive visits. GSS at Visit 02 (Day 30) was 11.52, Visit 03 (Day 60) was 10.29, Visit 04 (Day 90) was 8.66 and finally at Visit 05 (Day 120) it was decreased to 7.00 (categorized as moderate). The GSS decreased progressively over the course of the study, indicating a positive effect of the test product on reducing hair graying severity with continued usage. The data analysis revealed a substantial improvement in the average GSS by 46.28% (P-value <0.01) on Day 120, when compared to baseline. This significant improvement strongly suggests that the test product was effective in producing an anti-gray effect on the hair (Table 2).
|
Parameter |
Descriptive Statistics |
Baseline Visit 01 (Day 01) |
Visit 02 (Day 30) |
Visit 03 (Day 60) |
Visit 04 (Day 90) |
Visit 05 (Day 120) |
|
|
|
N |
29 |
29 |
28 |
29 |
29 |
|||
|
Mean |
13.03 |
11.52 |
10.29 |
8.66 |
7 |
|||
|
SD |
1.88 |
2.1 |
1.86 |
2.33 |
2.48 |
|||
|
Gray Severity Score (GSS) |
Median |
14 |
11 |
10 |
9 |
6 |
||
|
Min |
8 |
8 |
6 |
5 |
5 |
|||
|
Max |
15 |
15 |
15 |
15 |
14 |
|||
|
%CFB |
- |
11.66% |
20.80% |
33.62% |
46.28% |
|||
|
P-value |
- |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
|||
|
N : Number of Subjects SD : Standard Deviation %CFB : Percentage Change from Baseline |
|
|||||||
Table 2 Change in Gray Severity Score (GSS) from Baseline (Day 01) to Visit 05 (Day 120)
Improvement in Anti-Graying of Hair was obtained by Image-Pro software, which showed an improvement in the anti-graying effect of the test product. The L* value, which is associated with the white and black color spectrum, decreased by 6.19% on Day 120. A decrease in the L* value indicates a reduction in the lightness of the hair, which means that the hair became less white and potentially more black or darker (Table 3).
|
Parameter |
Descriptive Statistics |
Visit 01 (Baseline) |
Visit 05 (Day 120) |
|
N |
29 |
29 |
|
|
Mean |
44.38 |
41.63 |
|
|
SD |
4.95 |
7.24 |
|
|
L* Value |
Median |
44.2 |
42.78 |
|
Min |
32.98 |
24.41 |
|
|
Max |
55.52 |
57.25 |
|
|
%CFB |
- |
6.19% |
|
|
P-value |
- |
0.12 |
|
|
N |
29 |
29 |
|
|
Mean |
106.32 |
99.28 |
|
|
Colour Intensity |
SD |
12.23 |
17.5 |
|
Median |
106.08 |
101.74 |
|
|
Min |
76.98 |
58.57 |
|
|
Max |
133.4 |
135.76 |
|
|
%CFB |
- |
6.23% |
|
|
P-value |
- |
0.1 |
|
|
L* Value- Lightness Value N- Number of Subjects SD- Standard Deviation %CFB- Percentage Change from Baseline |
|||
Table 3 Improvement in Anti-Graying of Hair by Image-Pro Analysis
Figure 1 indicates change in gray hair before and after test product usage obtained by Image Pro analysis (Subject# 001) and Figure 2 indicates change in gray hair at right temporal part of head before and after test product usage obtained by CASLite-Nova (Subject # 006) (post written informed consent). The result of pluck test indicated a significant improvement in the anagen/telogen ratio of hairs. During the baseline visit (Day 01), it was observed that 67.50% of the hairs were in the anagen stage while 32.88% were in the telogen stage, resulting in an A:T ratio of 2:1. However, after 120 days of treatment, there was a significant enhancement in hair growth, with 86.57% (P-value <0.01) of hairs in the anagen stage and 13.41% (P-value <0.01) in the telogen stage, leading to an impressive A:T ratio of 6:1 (Table 4). Figure 3 (Subject# 001) indicates real pictures (post written informed consent) captured during study which shows increase in anagen to telogen ratio before and after test product usage.
|
Parameter |
Descriptive Statistics |
Baseline Visit 01 (Day 01) |
Visit 05 (Day 120) |
|
Mean |
8.28 |
6.83 |
|
|
SD |
2.19 |
1.61 |
|
|
Total Hair Count (N = 29) |
Median |
8 |
7 |
|
Min |
5 |
4 |
|
|
Max |
12 |
9 |
|
|
P-value |
0.01 |
||
|
Mean |
5.66 |
5.86 |
|
|
SD |
1.9 |
1.36 |
|
|
Anagen Hairs (N = 29) |
Median |
6 |
6 |
|
Min |
3 |
3 |
|
|
Max |
9 |
8 |
|
|
P-value |
0.63 |
||
|
Mean |
2.62 |
0.97 |
|
|
SD |
0.9 |
0.78 |
|
|
Telogen Hairs (N = 29) |
Median |
3 |
1 |
|
Min |
0 |
0 |
|
|
Max |
4 |
3 |
|
|
P-value |
<0.01 |
||
|
Mean |
67.5 |
86.57 |
|
|
SD |
10.5 |
10.15 |
|
|
Percentage of Anagen Hairs (%) (N = 29) |
Median |
66.66 |
85.71 |
|
Min |
50 |
66.66 |
|
|
Max |
100 |
100 |
|
|
P-value |
<0.01 |
||
|
Mean |
32.88 |
13.41 |
|
|
SD |
10.73 |
10.15 |
|
|
Percentage of Telogen Hairs (%) (N = 29) |
Median |
33.33 |
14.28 |
|
Min |
0 |
0 |
|
|
Max |
50 |
33.33 |
|
|
P-value |
<0.01 |
||
|
N- Number of Subjects SD- Standard Deviation %CFB- Percentage Change from Baseline |
|||
Table 4 Descriptive Statistics for Pluck Test from Baseline Visit 01 (Day 01) to Visit 05 (Day 120)
In terms of general appearance of hair, the results at Day 120 showed that 100% of the subjects experienced significant improvements in various hair attributes after using the test product. Specifically, all subjects (100%) reported enhancements in hair density, hair shininess, and hair smoothness. Moreover, none of the subjects felt any oily residue in their hair, indicating that the test product did not cause any greasiness or oiliness after usage. These findings demonstrate the positive and comprehensive effects of the test product on overall hair quality and appearance (Table 5).
|
Parameter |
Interpretation |
Baseline Visit 01 (Day 01) |
Visit 02 (Day 30) |
Visit 03 (Day 60) |
Visit 04 (Day 90) |
Visit 5 (Day 120) |
|
N=29 |
N=29 |
N=28 |
N=29 |
N=29 |
||
|
Small |
16(55.17%) |
16(55.17%) |
3(10.34%) |
1(3.45%) |
0(0.00%) |
|
|
Hair Volume |
Medium |
12(41.38%) |
9(31.03%) |
15(51.72%) |
7(24.14%) |
1(3.45%) |
|
Full |
1(3.45%) |
4(13.79%) |
10(34.48%) |
21(72.41%) |
28(96.55%) |
|
|
Hair Reflection |
Shiny |
2(6.90%) |
10(34.48%) |
18(62.07%) |
21(72.41%) |
27(93.10%) |
|
Blunt |
27(93.10%) |
19(65.52%) |
10(34.48%) |
8(27.59%) |
2(6.90%) |
|
|
Hair Plasticity |
Waved |
9(31.03%) |
9(31.03%) |
8(27.59%) |
10(34.48%) |
10(34.48%) |
|
Flat |
20(68.97%) |
20(68.97%) |
20(68.97%) |
19(65.52%) |
19(65.52%) |
|
|
Hair Density |
Dense |
3(10.34%) |
5(17.24%) |
13(44.83%) |
26(89.66%) |
29(100.00%) |
|
Thinned /Shed |
26(89.66%) |
24(82.76%) |
15(51.72%) |
3(10.34%) |
0(0.00%) |
|
|
Hair Smoothness |
Poor |
23(79.31%) |
9(31.03%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
Average |
6(20.69%) |
20(68.97%) |
15(51.72%) |
2(6.90%) |
0(0.00%) |
|
|
Good |
0(0.00%) |
0(0.00%) |
13(44.83%) |
27(93.10%) |
29(100.00%) |
|
|
Hair Oiliness |
None |
11(37.93%) |
10(34.48%) |
20(68.97%) |
26(89.66%) |
29(100.00%) |
|
Mild |
13(44.83%) |
15(51.72%) |
8(27.59%) |
3(10.34%) |
0(0.00%) |
|
|
Moderate |
5(17.24%) |
4(13.79%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
|
Hair Shininess |
Poor |
26(89.66%) |
8(27.59%) |
1(3.45%) |
0(0.00%) |
0(0.00%) |
|
Average |
3(10.34%) |
21(72.41%) |
9(31.03%) |
1(3.45%) |
0(0.00%) |
|
|
Good |
0(0.00%) |
0(0.00%) |
18(62.07%) |
28(96.55%) |
29(100.00%) |
|
|
N = Number of Subjects |
||||||
Table 5 Count & Percentage of General Appearance of Hair from Baseline (Day 01) to Visit 5 (Day 120)
Results of general appearance of scalp showed that there was no worsening of scalp condition reported throughout the study period of 120 days. Compared to baseline Day 01, none of the subjects had complaints of scalp itchiness, scalp redness, scalp roughness, and scalp scaliness at Day 120, clearly indicating the efficacy of the study product (Table 6).
|
Parameter |
Interpretation |
Baseline Visit 01 (Day 01) |
Visit 02 (Day 30) |
Visit 03 (Day 60) |
Visit 04 (Day 90) |
Visit 5 (Day 120) |
|
N=29 |
N=29 |
N=28 |
N=29 |
N=29 |
||
|
Scalp Itchiness |
None |
11(37.93%) |
16(55.17%) |
22(75.86%) |
29(100.00%) |
29(100.00%) |
|
Mild |
11(37.93%) |
10(34.48%) |
6(20.69%) |
0(0.00%) |
0(0.00%) |
|
|
Moderate |
7(24.14%) |
3(10.34%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
|
Scalp Redness |
None |
18(62.07%) |
20(68.97%) |
28(96.55%) |
29(100.00%) |
29(100.00%) |
|
Mild |
11(37.93%) |
9(31.03%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
|
Moderate |
0(0.00%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
|
Scalp Roughness |
None |
0(0.00%) |
4(13.79%) |
18(62.07%) |
27(93.10%) |
29(100.00%) |
|
Mild |
12(41.38%) |
18(62.07%) |
10(34.48%) |
2(6.90%) |
0(0.00%) |
|
|
Moderate |
13(44.83%) |
6(10.69%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
|
Excessive |
4(13.79%) |
1(3.45%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
|
|
Scalp Scaliness |
None |
17(58.62%) |
16(55.17%) |
26(89.66%) |
29(100.00%) |
29(100.00%) |
|
Mild |
8(27.59%) |
9(31.03%) |
2(6.90%) |
0(0.00%) |
0(0.00%) |
|
|
Moderate |
4(13.79%) |
4(13.79%) |
0(0.00%) |
0(0.00%) |
0(0.00%) |
Table 6 Count & Percentage of General Appearance of Scalp from Baseline (Day 01) to Visit 5 (Day 120)
Significant improvement was observed in hair strength with 100% of the subjects showing improved hair strength at Day 120 after the usage of test product.
Subjects’ perception regarding the test product was measured using a Subject Perception Questionnaire before and after the test product usage. Based on subjects’ perception questionnaire it was observed that at Day 01, 65.5% of subjects had moderate hair frizziness, 86.2% subjects had no irritation such as redness, dryness, itchiness, burning sensation of the scalp with their previous hair fall controlling product, 62.1% subjects felt average hair combing ability, 48.3% subjects had some extent of shininess in their hair, 51.7% of subjects had soft hair to some extent, and 69% subjects had moderate quantity of gray hair (Figure 4). After the usage of test product, the results improved with each successive visit with best results showed at Day 120. At Day 120, all the subjects felt improvement in hair frizziness, shininess, softness and silkiness with none of the subjects feeling any redness, dryness, itchiness or burning sensation of the scalp with the following test product proving the well tolerability of the product. 100% of the subjects felt very good about their hair combing ability and were satisfied to large extent with the study product. All the subjects felt that the test product was very much effective in reducing the gray hairs (Figure 5).
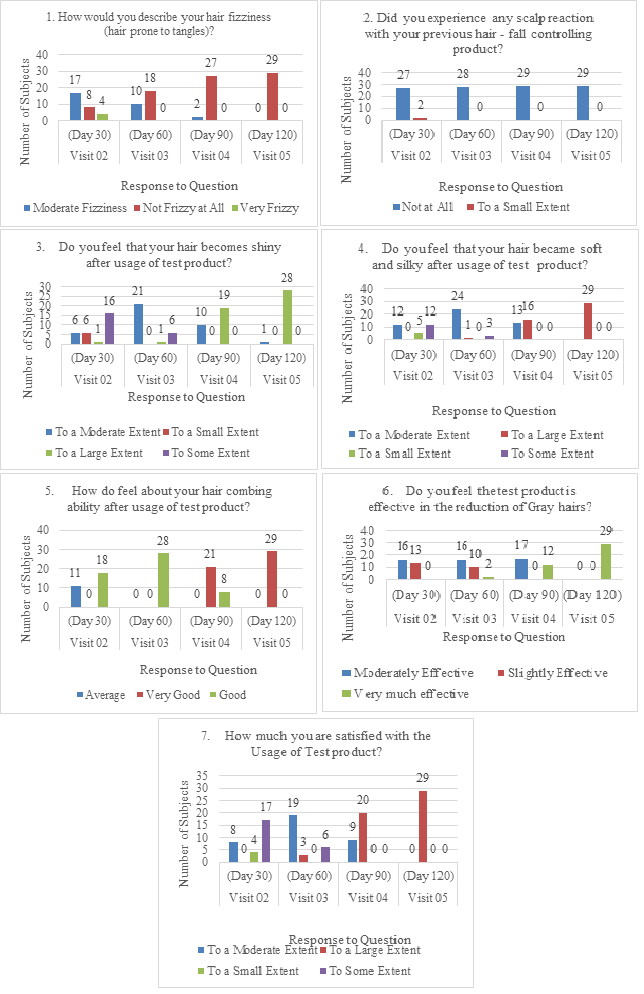
Figure 5 Subject perception questionnaire after test product usage at Visit 02 (Day 30 to Visit 05 (Day 120).
Furthermore, the test product was proved to be safe to use as there were no adverse events reported by neither the subjects nor the Investigator during the conduct of the study. From the derived results it can be said that the test product was safe and effective in the reduction of gray hairs in healthy adult human subjects with partially gray hairs.
This study provides valuable insights on anti-gray hair serum containing active ingredients like Arcedin™ [Aqua and Propanediol, Citrus Reticulata Extract/Citrus Reticulata (Tangerine) Extract and Acetyl Tyrosine and Pentylene Glycol and Gluconolactone and Sodium Benzoate and Aqua/Water and Biotin], Aloe barbadensis Extract, Polyquaternium-7, Camellia Sinensis Leaf Extract, Panthenol, and Rosmarinus officinalis in the reduction of gray hair and overall improvement in general appearance of hair and scalp in healthy human subjects having gray hairs. Till date there are very few studies that have been done in hair serum containing Aqua and Propanediol, Citrus Reticulata Extract/Citrus Reticulata (Tangerine) Extract and Acetyl Tyrosine and Pentylene Glycol and Gluconolactone and Sodium Benzoate and Aqua/Water and Biotin, making our study a highly contributing source in the field of dermatology.11
Hair dyes are affordable and easy to use substance for colouring of gray hairs. However, they are associated with side effects like irritation of the scalp, allergic reactions, and damage to the hair shaft.12 Here comes the role a hair serum that effectively reduces gray hair without causing major side effects. The test product is one such product which is safe and effective for use in healthy human subjects with gray hairs. The test product contains biotin which helps in healthy growth and strengthening of nails and hairs.12,13
Furthermore, it also helps in the treatment of hair loss.12 Arcolys is another ingredient present in our product. It is a natural ingredient which is derived from plant called Picrorhiza scrophulariiflora. R Boutin et al.,14 conducted a placebo-controlled study involving 29 females and 15 males with 20 to 50% white hairs to study the effects of Arcolys on oxidative stress and melanogenesis pathway. Results of the study showed that Arcolys enhances melanogenesis and increases production of melanin inducing pigmentation of hairs and a significant decrease in the white hair density. Moreover, 80% of the females reported a good effect of Arcolys on their hair re-pigmentation. The results of this study are in line with our study indicating a significant decrease in gray hairs and GSS score with the use of hair serum containing Arcolys.14 Melanogray is an award-winning ingredient launched by Mibelle Biochemistry which is an anti-hair graying essence which is obtained by upcycling the peel paste from Chios mandarians. It works by stimulating melanin and antioxidant activity thereby, reducing the quantity of gray hair in both males and females. Study done by Mibelle Biochemistry suggests that that Melanogray reduces gray hairs by 21.6% in both the genders and possesses long-lasting re-pigmentation effect.6
The novelty of the test product is the combination of Rosmarinus officinalis with advanced hair melanin synthesizing ingredients that is responsible for the maximum impact on the premature graying of hair. The high concentration of phenolic components in Rosemary like rosmarinic acid and carnosic acid creates a potent shield against oxidative stress, which is a common factor in premature graying. Unlike any other Anti-graying hair serum, Rosemary synergistically works with Arcedin™ [Aqua and Propanediol, Citrus Reticulata Extract/Citrus Reticulata (Tangerine) Extract and Acetyl Tyrosine and Pentylene Glycol and Gluconolactone and Sodium Benzoate and Aqua/Water and Biotin] and potentially leads to more noticeable and lasting results in reducing gray hair.
The mixture of all these ingredients works as an effective anti-gray hair product in population having gray hair along with complaints like scalp itchiness, redness, dryness and burning sensation. Absence of product related adverse events makes it a safe and well tolerable product for its utilization in targeted population. There are certain limitations to our study. The study has been conducted on small number of subjects. Study involving larger number of subjects is required to study efficacy and safety in larger groups. One key challenge encountered by study physician/dermatologist was the assessment of the patient adherence to the test product and shorter study duration. Moreover, the study lacks comparative nature with placebo or similar substances.
The results obtained from this study hold substantial promise for advancing our scientific comprehension of how hair serums can be effectively employed to address the issue of graying hair. The incorporation of the active ingredient Arcedin™ has demonstrated its efficacy and well tolerability in effectively reversing the graying of hair after a 120-day period of continuous use of the test product. In essence, the research not only sheds light on the mechanisms behind graying hair reversal but also underscores the broader utility of the test product as a comprehensive solution for enhancing hair and scalp health, thereby offering valuable insights into the realm of hair care science, thereby reaffirming its merit as a valuable addition to daily hair care regimens.
The authors are grateful to Anveya Living Private Limited and its team for their kind collaboration. The authors are also thankful to the NovoBliss Research (https://novobliss.in) study team for the in-vivo clinical study conduct, the statistical team for data analysis, and the scientific writing team for support in the preparation of this manuscript. We extend our thanks to Ms. Megha Yadav as Assistant Project Manager, Ms. Nistha Jani for the overall coordination of the clinical study and Dr. Dhruvil Gajera as Medical Writer. The authors also thank all the subjects who participated in this study.
Authors declare there is no conflict of interest.

©2023 Merja, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.