Journal of
eISSN: 2574-9943


Literature Review Volume 6 Issue 2
1Department of Dermatology, The Third Affiliated Hospital of Soochow University, China
2Department of Dermatology, The First Affiliated Hospital of Bengbu Medical University, China
Correspondence: Ru-Zhi Zhang, Department of Dermatology, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, 213003
Received: June 23, 2022 | Published: July 12, 2022
Citation: Huang T, Jin H, Zhang R. Research progress towards skin repigmentation in vitiligo. J Dermat Cosmetol. 2022;6(2):46-48. DOI: 10.15406/jdc.2022.06.00207
Vitiligo is a common skin disease that results from depigmentation of the skin and mucous membranes due to the destruction of functional melanocytes (MCs). Vitiligo is caused by the interactions of multiple susceptibility genes and acquired immune-related factors. The autologous transplantation of MCs is one of the most effective surgical treatment options for patients with refractory quiescent vitiligo who do not respond to medical treatment and phototherapy. At present, cell suspension transplantation still faces many challenges, including damage to MCs during the operation, failure to ensure the adhesion of MCs at the recipient area, and a poor cell survival environment within a short period of time after transplantation. Therefore, the transplantation of MC patches has significant advantages. Chitosan can not only promote the adhesion, proliferation and migration of MCs, but also induces MCs to form spheroids, which greatly improves the activity of MCs and improves the repigmentation rate of the skin after transplantation. The properties of the matrix material used and the density of seeded MCs influence the process of spheroid formation. In the future, it is necessary to develop better MC patches to improve the success rate of vitiligo treatment.
Keywords: vitiligo, chitosan, melanocyte, patches, spheroid
Vitiligo is the most common depigmented skin disease and is caused by the autoimmune destruction of functional epidermal melanocytes (MCs).1,2 Patients with vitiligo develop a psychological sense of social shame due to the presence of depigmented spots that impair their appearance. Cell transplantation is usually required for quiescent vitiligo lesions that do not respond to medical treatment and phototherapy.3 For larger lesions, autologous MCs cultured in vitro are usually transplanted.4 The procedure is as follows: the epidermis at the recipient area is removed with a grinder or laser, after which the MCs are cultured in vitro, collected and prepared in a cell suspension, which is spread evenly on the epidermis-free recipient area and then covered with a dressing. As the transplanted MCs survive and proliferate, the vitiliginous skin begins to repigment. However, the transplantation of MC suspensions has drawbacks that affect the therapeutic efficacy. In order to improve the therapeutic efficacy, melanocyte patches (MPs) have been developed to replace the MC suspension and this article reviews recent studies on MPs.5
Many factors impair the viability of MCs transplanted in cell suspensions, including:5,6 (1) Before transplantation, preparation of the cell suspension using trypsin digestion and mechanical shaking may damage MCs and cause them to undergo apoptosis or death. (2) During the transplantation process, it cannot be ensured that MCs in the suspension will adhere tightly to the recipient area, especially when the surface of that area is uneven.6 (3) The concentration and volume of the cell suspension is difficult to control. When the liquid volume is large and the cell concentration is low, the cell suspension will flow out of the recipient area and accumulate in depressions in that area. On the contrary, when the liquid volume is small and the cell concentration is high, the affected area cannot be completely covered, and the cell suspension can quickly dry up, affecting the activity of the cells. (4) The cell survival environment in the recipient area is harsh. The recipient area of the exfoliated epidermis is initially devoid of keratinocytes and lacks exogenous trophic factors from keratinocytes that favor the survival of MCs.6
In view of the unfavorable factors of cell suspension transplantation listed above, scientists and clinicians have tried to implant MCs on biological materials to form MPs, which could be directly used for transplantation in the treatment of vitiligo. Therefore, handling of cells prior to transplantation is no longer required, and thereby avoids damage to the cells caused by trypsin digestion and mechanical shaking. The application of MPs will save time for adding cells to the recipient areas. Transplantation of MPs ensures that MCs are tightly attached to the area even if the surface of the recipient area is uneven. In highly mobile areas, MPs can be secured to the skin with dressings, bandages or sutures. The biomaterial used in MPs not only provides more favorable living conditions for the transplanted cells, but also promotes epithelialization and shortens the recovery period.7 The antimicrobial properties of biomaterials can reduce the risk of secondary infections. It has been reported that MCs within MPs were successfully transferred to the depigmented areas of nude mice and survived well.5 MPs were transplanted onto the epidermal wounds of nude mice and were active.
Biomaterials as basement membranes
Chitosan (CS) membranes
The key point of MP preparation is to select suitable support materials. To date, the most studied support material used is chitosan (CS). CS possesses some favorable properties including biodegradability, non-toxicity, good biocompatibility, easy availability and economy. CS has been widely used in medicines, foods, biochemicals and other fields.8,9 The surface of CS is flat and transparent, which facilitates the observation of cell behavior and the timely detection of possible contamination. In addition, CS has high water absorption and good antibacterial properties.10,11 In addition to these features, the most valuable characteristic is that CS can induce the formation of MC spheroids (MSs), which is very important for the successful treatment of vitiligo6 (Figure 1). In 2005, a research team from the Institute of Biomedical Engineering, National Taiwan University reported for the first time that MCs seeded on CS grew into aggregates of cells, termed MSs, which are more viable than monolayer cells in harsh environments lacking growth factors.12 The formation of MSs on biomaterials depends on the competition of cell-cell interactions and cell-matrix interactions,12 and when the former exceeds the latter, the cells may migrate and aggregate into spheroids.7 Conversely, the cells will appear in a monolayer pattern.
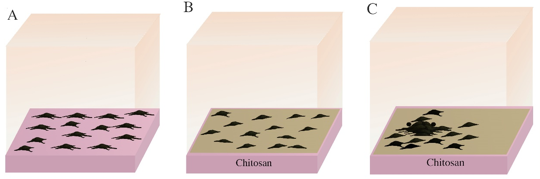
Figure 1 The process of culturing melanocytes into dense aggregates using chitosan under specific conditions. A, primary culture of cells; B, melanocytes seeded on chitosan membrane; C, formed spheres .
Polyvinyl alcohol (PVA) membranes
Polyvinyl alcohol (PVA) membranes are biomaterials of hydrophilic polymers. It has been reported that PVA induces several distinct types of cells to grow as spheroids, including neural stem cells and acinar cells.13-15 However, the mechanisms by which CS and PVA induce cells to form spheroids are different. MCs at the same density were seeded onto CS and PVA membranes. Those cells in the PVA group appeared round 2 h later, remained suspended and then aggregated into multiple MSs within 24 h. The suspended MSs further aggregated into larger spheres by day 3. On days 5 to 7 of culture, the spheroids become smaller and cell debris was observed in the suspension, suggesting cell death and the disintegration of MSs. After 9 days of culture, the viability of cells within the PVA-induced MSs dropped to 44%, compared to more than 90% for CS-induced MSs. On the contrary, MCs in the CS group attached to CS membranes in a spindle-shape within 2 h after inoculation, and took on a typical dendrite shape and began to aggregate 24 h later. During 3-7 d of culture, MSs became more compact, and some dendritic MCs appeared between MSs after 7 d. Therefore, those studies speculated that CS simultaneously promotes cell-cell and cell-substrate interactions, resulting in aggregation, while PVA-induced MSs mainly depended on random cell-cell collisions in the suspension.16
Nylon-CS mixed membranes
CS has been widely used in tissue engineering.8 However, CS has a relatively low mechanical strength for clinical use. Scientists tried to prepare a mixed membrane, which not only maintains the property of CS, but also enhances its mechanical strength. Nylon was chosen as one component in the mixed membrane (namely, nylon–chitosan-blended membranes, NCBM) due to its adhesion.7 Nylon and CS in the NCBM do not react chemically, each maintains its own properties.17 Different composition ratios of these two substances have different effects on the formation, adhesion and migration of MS.
CS membranes are dense and flat, while nylon membranes are characterized by particle surfaces. As the proportion of nylon increasing, the surface of NCBMs gradually becomes uneven. MCs on NCBMs containing 25% nylon (NC75) or on pure CS membranes showed bipolar morphology with short dendrites. The surface of NCBMs containing nylon 50% (NC50) remains roughly flat, on which the MCs do not aggregate due to their elongated dendrites spanning multiple nylon particles. MCs on NCBMs containing nylon 75% (NC25) or pure nylon membranes are mainly located in the low-lying areas at 4 h after seeding, but eventually occupy the entire surface of the NCBM on the 7th day of culture, suggesting that the undulation of the membrane surface does not prevent cell migration and growth, namely, the undulating surface does not affect the formation of MSs.
Better adhesion of MCs to nylon may reflect stronger cell-substrate interactions, which inhibit the formation of MSs. The interaction between a single cell and a substrate is the sum of cell-substrate interactions at different contact points. MCs have longer dendrites and more contact points with nylon. With increasing nylon content in the NCBM, the cell-substrate interactions are enhanced and exceed the cell-cell interactions, the formation of MSs is inhibited. Previous studies found that the growth of MCs on NC75 membranes is enhanced, on NC50 membranes is the highest and on NC25 membranes is reduced compared to those growing on pure CS membranes. The opacity of NCBMs is proportional to their nylon content. Only pure CS membranes and NC75 membranes allow direct observation with an inverted microscope, and for clinical applications, the transparency of the membrane is advantageous. Therefore, scientists are working to find the most appropriate nylon content in NCBMs.
Cell adhesion and migration on material membranes
Cell adhesion is the interaction of a cell with the extracellular matrix (ECM), mediated by multi-protein adhesion structures such as focal adhesions, fibrillar adhesions and podosomes.18 Cell adhesion is a multi-step process involving the adsorption of ECM proteins onto the substrate surface, the recognition of ECM components by cell surface receptors, and the subsequent rearrangement of the cytoskeleton leading to cell adhesion.19 Previous studies indicated that fibronectin (FN) plays an important role in the adhesion, migration and aggregation of MCs. The adsorption of FN from serum-containing media to the surface of biomaterials plays a crucial role in mediating cell adhesion. CS and PVA membranes were incubated in fresh medium (containing 10% fetal bovine serum), and the adsorption of FN on the surfaces of CS and PVA membranes was assessed by western blotting. The results showed that the amount of FN adsorbed on CS membranes was significantly higher than that on PVA membranes. In the presence of anti-FN antibodies, the ability of MCs to form MSs was reduced. FN-depleted medium almost completely inhibited the formation of MSs on CS membranes, thus suggesting that FN plays a key role in the formation of MSs. Another study found that cadherin synergistically enhances cell adhesion.20
Cell density affects the formation of MSs
Depending on the seeding density and culture time, MCs cultured on biomaterials grow as a monolayer or in a spherical pattern. When the seeding density of cells exceeds 10×103cells/cm2, the MCs began to aggregate on the CS surface within two days of culture and grew into more dense MSs on the 3rd day. A higher seeding density results in more MS formation in less time.5,12 A lower cell density means larger cell-to-cell distances, which results in relatively weak intercellular interactions, and strong interactions of cell-biomaterial substance. Therefore, the MCs grow in monolayers and rarely form MSs. Whether or not MSs can be formed, and how long that takes, depends on the initial seeding density of cells. Given sufficient time in culture, cells will eventually form spheroids even at low seeding densities. Another possible mechanism for the formation of MSs is that when the cell density reaches a critical concentration,6 MCs begin to secrete soluble factors that can promote their aggregation and promote the formation of MSs.
None.
The author declares there is no conflict of interest.

©2022 Huang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.