Journal of
eISSN: 2574-9943


Case Report Volume 6 Issue 3
1Sector of Dermatology, Santa Teresa Hospital, Santa Catarina State Secretary for Health, Brazil
2Faculty of Medicine, South Santa Catarina University, Brazil
3Polydoro Ernani de São Thiago University Hospital – Santa Catarina Federal University, Brazil
Correspondence: Bruna Molozzi, Antonio José Raulino Avenue, no number, Santa Teresa Colony, São Pedro de Alcântara, Santa Catarina, Brazil, ZIP-Code: 88125-000, Tel (48) 996561966
Received: June 06, 2022 | Published: July 25, 2022
Citation: Molozzi B, Paul IR, Dorn TV, et al. Photosensitivity and cholestatic hepatitis: the dermatologist contribution for the diagnosis of erythropoietic protoporphyria. J Dermat Cosmetol. 2022;6(3):60-62. DOI: 10.15406/jdc.2022.06.00211
We report a case of erythropoietic protoporphyria (EPP) in a young male adult, who was admitted to the hospital due to abdominal pain and jaundice. Complementary exams showed elevation of liver and canalicular enzymes and choledocholithiasis. The patient also had pronounced photodamaged skin and a history of significant photosensitivity since childhood. The dermatology team, based on the clinical and histological findings, gave the diagnosis of EPP and treatment with cholestyramine was instituted.
Keywords: photosensitivity, porphyria, heme-biosynthesis, erythropoietic protoporphyria, ferrochelatase, choledocholithiasis
Erythropoietic protoporphyria (EPP) is a rare autosomal recessive disorder of heme biosynthesis, caused by mutations in the ferrochelatase (FECH) gene.1,2 Over 190 mutations have been reported in the FECH gene including missense, nonsense, splicing and frameshift mutations.3 FECH is the final enzyme in the heme-biosynthetic pathway, responsible for the insertion of iron into protoporphyrin IX (PPIX) to generate the final product heme, and its reduced activity leads to the accumulation of PPIX, a phototoxic compound. PPIX in skin and vessels can absorb light energy and enter an excited energy state, resulting in oxygen radicals that produce tissue and vessel damage.2,4 EPP is 100% penetrant, and is the most common porphyria in children. Males and females are equally affected, and the main symptoms are painful photosensitivity, early photodamage and hepatobiliary changes.3 The aim of this work is to report a case of EPP, drawing attention to this entity, highlighting the contribution of the dermatological findings for the diagnosis.
A 30-year-old male, alcoholic, sought medical care at Hospital Santa Teresa, (located in the city of São Pedro de Alcântara, state of Santa Catarina, Brazil) due to abdominal pain and jaundice. He referred to pain in the upper right abdomen, nausea, vomiting and clay-colored stools. Complementary exams showed increased erythrocyte sedimentation rate (42 mm), mixed hyperbilirubinemia (total bilirubin: 20 mg/dL), increased transaminases (aspartate aminotransferase: 130 U/L, alanine aminotransferase: 268 U/L) and canalicular enzymes (gamma-glutamyl transferase: 649 U/L, alkaline phosphatase: 238 U/L). Serologies for HIV, hepatitis C and B were negative. Abdominal ultrasound showed choledocholithiasis.
Dermatological examination showed pronounced photodamaged skin, noticed by a thick, leathery, opaque skin, with deep wrinkles, predominating in the glabella and ears. On the neck, there was cutis rhomboidalis nuchae (Figure 1). On the back of the hands and feet there were numerous non-coalescent tender papules with smooth surface (Figure 2). He reported burning sensation and sometimes severe skin pain after minimal sun exposure, since he was a kid. His brother had similar dermatological findings.
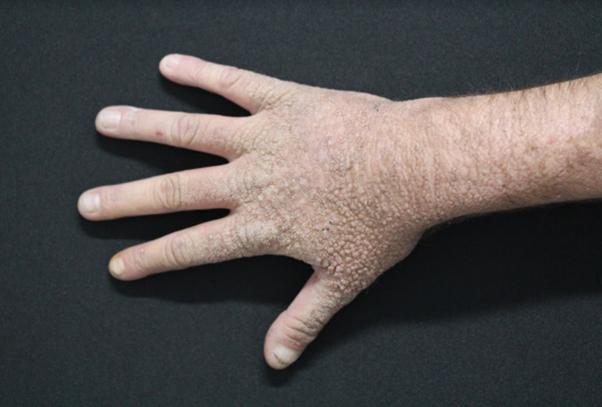
Figure 2 Numerous non-coalescent tender papules with smooth surface on the back of the hand and feet.
Porphyria was our main hypothesis, although porphyrins urine test was negative and no fluorescence was detected on the urine. Subsequently, a skin biopsy was performed, which revealed hyaline deposits in the papillary dermis and around the vessels that became more evident on Periodic Acid-Schiff (PAS) stain with diastase (Figure 3 & Figure 4). Immunohistochemistry was also performed, and revealed positivity for type IV collagen (Figure 5).
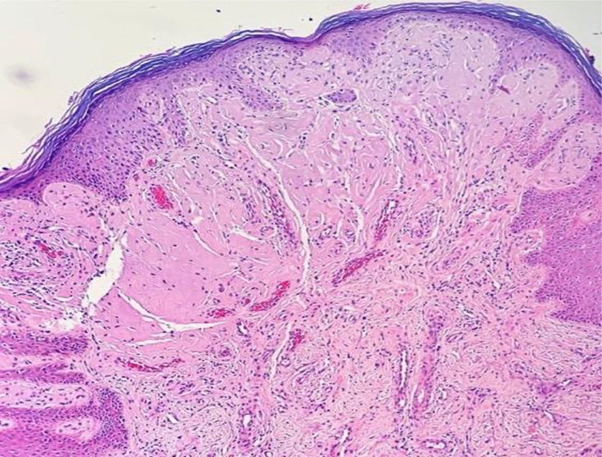
Figure 3 Histological skin sections show deposits of hyaline material in the papillary dermis and around vessels (hematoxylin & eosin).
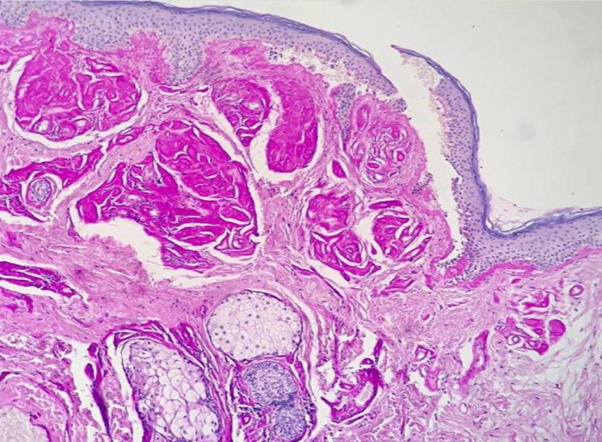
Figure 4 Hyaline deposits in the papillary dermis and around the vessels that became more evident on Periodic Acid-Schiff (PAS) stain with diastase.
Combining the patient's history, physical examination and histological findings, the diagnosis of EPP was made. Cholecystectomy surgery with a choledochal approach was performed; oral treatment with cholestyramine was initiated, in addition to strict photoprotection and alcoholism cessation. The dermatology team and the hepatology team are currently following him.
EPP causes painful cutaneous phototoxicity within minutes of sun exposure, usually starting in infancy. The mean age of symptom onset is about 4 years.1–4 The pain is usually preceded by prodromal symptoms, such as tingling, itching, and burning sensation on the skin. In case of prolonged exposure, erythema or edema can appear.1,2 Bullous lesions, hirsutism, scarring or/and hyperpigmentation are not typically seen in EPP.4 Over time, patients develop behavior of sun avoidance, which has major implications for daily life, affecting family life, social life, and their ability to work.1,3,4
Protoporphyrin excess is excreted by the liver into the bile and can precipitate in hepatocytes and bile canaliculi, causing hepatotoxicity and increasing the risk of gallstones. Approximately 5% of patients develop significant liver dysfunction, and concomitant conditions such as viral hepatitis, excessive alcohol consumption, and use of drugs which induce cholestasis may contribute to worsening liver disease.1,3,4
The diagnosis of EPP is made by detection of increased levels of protoporphyrin in erythrocytes, plasma and feces, but not in urine, as it is not water-soluble. Genetic testing by sequencing the FECH gene can confirm its diagnosis.4,5 Histopathology shows that in the photoexposed skin there is a thickening in vessels walls of the papillary dermis due to the accumulation of an amorphous and homogeneous material, which is stained by PAS, as well as positivity to type IV collagen in Immunohistochemistry study.6 Previous reports suggest that the diagnostic delay can be over a decade. Difficulties in making the diagnosis include the lack of cutaneous lesions in between attacks and the absence of abnormalities on routine laboratory studies. In addition, protoporphyrin testing needs to be sent to a specialty laboratory for an accurate diagnosis.1,7
The mainstay of treatment of erythropoietic protoporphyria is sun avoidance and/or sun protection.4 As sunscreen only blocks UVA and UVB and does not block light in the visible spectrum such as blue light (>400 nm), conventional topical sunscreens are not effective in EPP. Many potential treatments for EPP have aimed at increasing skin pigmentation, in order to prevent blue light from penetrating the skin and activating PPIX.3 Oral beta-carotene has been used for many years to improve sun tolerance in patients. The dose depends on age and needs to be adjusted to maintain serum carotene at a level high enough to cause mild skin discoloration due to carotenemia.4,6,7 However, only one randomized double-blind placebo-controlled crossover trial was performed, which did not show a beneficial effect of beta-carotene on hours spent outdoors.3,8 Other drugs such as n-acetyl cysteine, cysteine and vitamin C have also been tried in EPP. Although, there are no data to support the efficacy of these treatments.3,4
Afamelanotide is a potent analogue of the human α-melanocyte stimulating hormone (α-MSH), which increases skin pigmentation by activation of eumelanin production. Performed studies concluded that afamelanotide exhibits good clinical effectiveness, good safety and offers an important improvement in increasing light exposure and quality of life in patients with EPP. Despite the outcomes, afamelanotide is still not available worldwide for the treatment of EPP.3,4,9 Furthermore, a new molecule and possible alternative treatment, MT-7117 (dersimelagon), is currently tested in a phase III trial, and can be a promising treatment.10
In case of liver disease, there are some treatment options. Bile acid sequestrants such as cholestyramine and other drugs such as ursodeoxycholelic acid have been used to increase the excretion of protoporphyrin through the biliary system.4 Liver transplant is the treatment for end-stage liver disease. Nevertheless, it is not curative, and the risk for disease recurrence in the graft is substantial because the excessive production of protoporphyrin by the bone marrow continues after liver transplantation.4,6,11 The only currently available curative option for EPP is allogeneic bone marrow transplantation. However, due to the risks of bone marrow transplantation, this is generally only performed in patients with severe EPP-associated liver disease.3,4,6
Erythropoietic porphyria is a rare disease, which still has a significant delay in its diagnosis. In this report, we showed the dermatologist's contribution to the diagnosis of the disease, reinforcing clinical aspects and current therapeutic options for EPP.
None.
Authors declare there is no conflict of interest.

©2022 Molozzi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.