Journal of
eISSN: 2574-9943


Case Report Volume 8 Issue 4
1Riga, Linove private practice, Latvia
2Riga, Academic Histology Laboratory, Latvia
3Riga, Riga Second Hospital, Department of Radiology, Latvia
Correspondence: Tatjana Linova, M.D, Riga Linove Private Practice, Latvia, Tel +371 28623244
Received: October 28, 2024 | Published: November 11, 2024
Citation: Linova T, Nevidovska K, Linovs V. Periorbital papules as a transient complication after type I collagen injections: a case report. J Dermat Cosmetol. 2024;8(4):103-105. DOI: 10.15406/jdc.2024.08.00276
This case report presents transient periorbital papule formation following type I collagen injections in three female patients aged 38-40 years, administered in the lower eyelid region for periorbital rejuvenation. Intradermal collagen injections, while promising for enhancing periorbital skin quality, carry a risk of localized reactions due to the region’s sensitivity. The patients reported non-painful, firm papules within 5-7 days post-procedure. High-resolution ultrasound confirmed hypoechoic collagen deposits adhering to the dermis, with histological examination in one patient revealing intradermal collagen clusters without surrounding inflammation. Two patients received PRP therapy and at-home massage, while the third was monitored remotely, showing spontaneous resolution within four weeks. Findings indicate that type I collagen injections can lead to transient papules, which resolve without visible scarring, supporting a favorable safety profile for this aesthetic intervention. Further studies are recommended to refine techniques and establish standardized protocols for periorbital collagen injections.
Keywords: collagen injections, collagen type I injections, periorbital rejuvenation
The aging of the periorbital region is closely associated with changes in skin quality. Aesthetic treatment options for rejuvenating the skin in this area are limited due to the prolonged recovery period and the restricted use of therapeutic agents in this sensitive region. Therefore, intradermal administration of type I collagen for aesthetic purposes has emerged as a promising therapeutic approach for this region.1 However, the periorbital area poses challenges, as it may lead to transient complications related to the introduction of foreign proteins and the provocation of rejection inflammatory reactions. This clinical case report describes three female patients who developed transient papules following intradermal administration of type I collagen in the skin of the lower eyelid. Patients were examined using a high-resolution ultrasound device, and a diagnostic skin biopsy was performed on one patient from the persistent papule in the periorbital region to assess the nature of the papule, its depth, and the type of inflammatory skin reaction. The papules resolved after four weeks without visible scarring.
This clinical case report describes three Caucasian female patients (Patient A, B, C), aged between 38-40 years, who underwent type I collagen intradermal injections in the skin of the periocular (lower eyelid) area in July 2022 in Dermatology clinic in Riga, Latvia. Written informed consent was obtained from each patient for the procedure and for the publication of anonymized data and images. This consent ensures that all patient rights were respected and that they were fully informed of potential risks and outcomes prior to treatment.The aim of the treatment was to reduce photoaging damage and improve overall skin quality. The patients were clinically healthy, with no reported allergies or medication intolerances. All three had previously undergone injections containing collagen peptides.
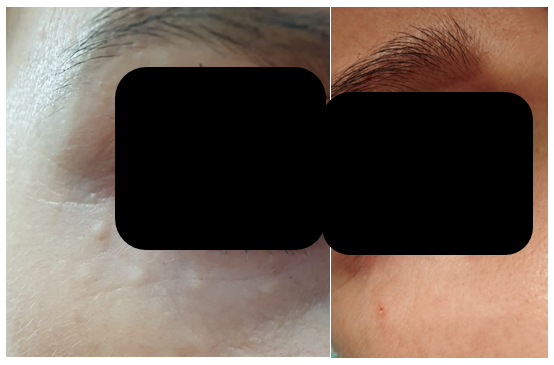
Figure 1 Patient A, a 39-year-old female: The image on the left, taken 14 days after intradermal administration of type I collagen, shows well-defined papules in the periorbital area of the right eye, with no visible signs of inflammation in the surrounding tissues. The papules were reported as painless and, upon palpation, presented as firm formations. The image on the right, taken on day 28 post-administration, shows complete resolution of the collagen deposits, with no visible evidence of papules remaining, aside from a minor biopsy scar.
All injections were performed using a micro-papular subdermal technique. NithyaÒ type I bovine collagen, manufactured by Euroresearch S.r.l. was used for these treatments. Each vial contained 70 mg of micronized collagen powder, which was reconstituted with 5 ml of sterile 0.9% saline solution, along with 0.3 ml of 2% lidocaine to reduce discomfort of procedure. The solution was administered in the periorbital (lower eyelid) area with a total injection volume of max 0.25 ml each side, using a 32 G, needle. Injection points were spaced approximately 1 cm apart to ensure even distribution around the target area. Each injection was angled at 15 degrees to the skin surface to deposit the collagen precisely within the superficial dermis.2
On days 5 and 7 following the procedure, the patients reported the persistence of micro papules in the periorbital areas. The papules were firm, non-painful, with minimal or no visible inflammatory reaction in the surrounding tissues. The patients A and B underwent follow-up consultations, massage and PRP (platelet-rich plasma) autologous injections were performed (Figure 1) (Figure 4). During consultation the status of the papules was evaluated using physical assessment with visual and palpatory control. The patients A and B underwent ultrasonographic examination on 13th day after collagen injection with a high-resolution 22 MHz Canon probe to determine the depth of the micro papules, which concluded the presence of the papules in subdermal layer. (Figure 2) (Figure 5A, B). The patient A underwent a tissue histology examination and 14th day after collagen injection to assess the nature of the papules, the reaction of the surrounding tissue, and the nature of the inflammation, which concluded that the papules are collagen collection without perifocal inflammation (Figure 3). Follow-up consultation was performed on day 28 after injection which concluded resolution of the symptoms, no visible or palpable papules were observed (Figure 5A & 5B).
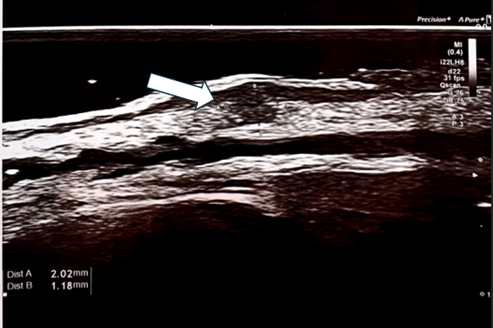
Figure 2 Ultrasonography of Patient A right eyelid skin, performed using a Canon Hockey Stick 22 MHz probe, revealed hypoechoic collagen aggregates closely adhering to the dermal layer.
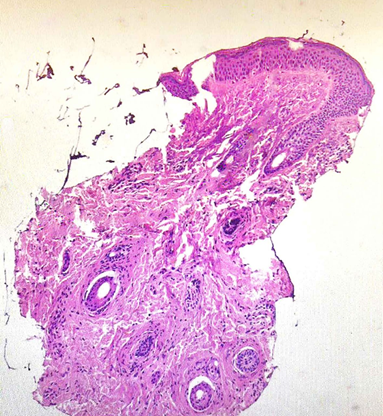
Figure 3 The histopathological evaluation of Patient A skin biopsy specimen from right eyelid skin, stained with haematoxylin and eosin (H&E), demonstrates a cluster of collagen fibres localized within the dermis, extending into the hypodermis, with no evidence of perilesional inflammatory infiltrate.
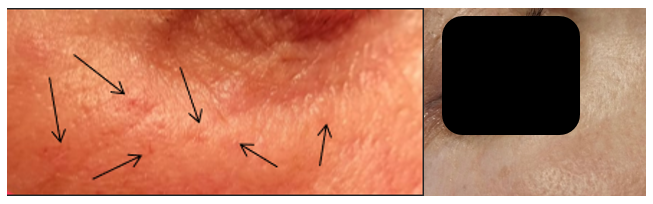
Figure 4 Patient B, a 40-year-old female: The image on the left, taken 14 days following intradermal administration of type I collagen, reveals mild papules, approximately 1 mm in size, in the periorbital area of the right eye. These papules were firm upon palpation, with no visible signs of inflammatory response in the surrounding tissues. The image on the right, taken on day 28 post-administration, shows no visible or palpable evidence of collagen deposits in the treated area, indicating full resolution of the papules.
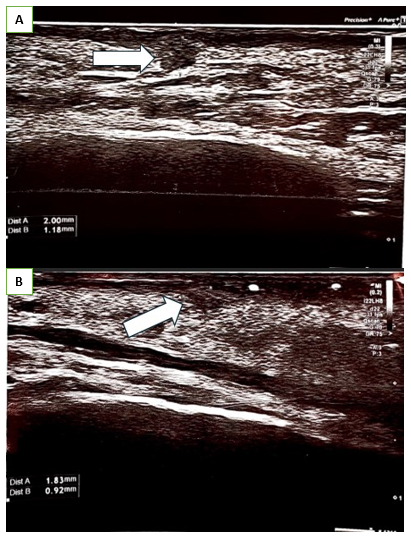
Figure 5 Ultrasonography of Patient B of right eyelid skin, performed using a Canon Hockey Stick 22 MHz probe, revealed hypoechoic collagen aggregates closely adhering to the dermal layer.
Patient C was monitored remotely without specific therapy. Patient C conducted self-assessments and sent weekly images of the periorbital region for review by the clinical doctor. Consultations were held via phone to discuss any changes, and the patient was instructed to document and report any symptoms. Despite not receiving additional interventions, Patient C experienced full papule resolution within 28 days, with no visible or palpable adverse effects noted in the follow-up assessments (Figure 6).
In all cases there was full resolution of papules on 28th day after the collagen injection in all patients which was observed clinically using visual control and palpation (Figure 6).
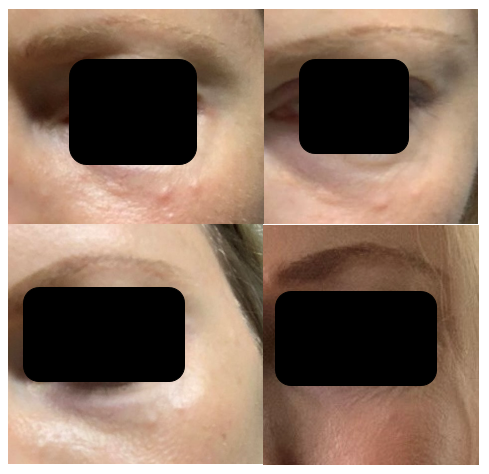
Figure 6 Patient C, a 38-year-old female: The initial image (upper left) was sent to the physician on day 7 following type I collagen injections, showing visible papules in the lower eyelid area. The subsequent images document the progression over time, with the upper right image taken on day 14, the lower left on day 21, and the final image on day 28. By the 28th day post-injection, the patient reported no visible or palpable papules in the treated area. Throughout this period, Patient C was monitored remotely, with regular photographic updates, and no additional therapeutic intervention was required.
The application of type I collagen injections in the periorbital region represents an innovative yet underexplored area in aesthetic dermatology. Unlike more established dermal fillers or botulinum toxin, intradermal collagen injections are relatively new for periorbital rejuvenation, and clinical data on their efficacy, safety, and potential complications are limited. This scarcity of information poses challenges in establishing standardized protocols and fully understanding the risks associated with collagen deposition in sensitive areas.
Most existing studies, such as the multicenter study by Sparavigna,3 have focused on the general safety profile of collagen injections, reporting improvements in skin texture, hydration, and elasticity with minimal adverse reactions.3 However, specific documentation on the localized effects, such as transient papule formation, in the periorbital region is sparse. Complications from collagen injections typically relate to the formation of nodules, hypersensitivity reactions, or granulomatous inflammation, yet these outcomes are predominantly associated with bovine collagen, which carries a slight risk of immunogenicity. Nithya collagen, being CE-certified and processed to minimize immune response, has demonstrated safety in other aesthetic areas, but controlled studies in periorbital applications are lacking.3
The findings in this case report contribute to the limited literature by documenting the transient formation of papules, suggesting a favorable tissue response. Nevertheless, the absence of long-term studies means that clinicians must approach these injections with caution, ensuring meticulous technique and close patient follow-up. The present cases highlight the need for more robust data to inform clinical guidelines and refine injection methods to minimize localized complications in sensitive regions like the periorbital area.
Further research, including randomized controlled trials and larger case series, is essential to define the optimal protocols, potential risks, and management strategies for type I collagen injections in the periorbital region. As more clinicians document their experiences and outcomes, the field will better understand the balance between efficacy and safety for this novel approach.
This case report underscores the need for further research into the potential complications associated with subdermal type I collagen injections, particularly in sensitive areas such as the periorbital region. Currently, there is limited data on the long-term safety, efficacy, and side-effect profile of these aesthetic treatments. Clinicians utilizing subdermal collagen injections should be informed of the possibility of transient papule formation, which, while typically self-resolving, may cause anxiety and dissatisfaction among patients. These findings emphasize the importance of patient education, meticulous technique, and careful follow-up to mitigate concerns and enhance patient satisfaction. Comprehensive studies are essential to establish standardized protocols and optimize outcomes for this aesthetic procedure.
None.
None.
The authors declared that there are no conflicts of interest.

©2024 Linova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.