Journal of
eISSN: 2574-9943


Research Article Volume 6 Issue 1
1Head Professor of the Post-Graduate Course of the ACA - Institute of Assistance in Plastic Surgery of São Paulo, Division of Plastic Surgery at the General Hospital São Rafael, Brazil
2Staff of the Post-Graduate Course of the ACA - Institute of Assistance in Plastic Surgery, Division of Plastic Surgery at the General Hospital São Rafael, Brazil
Correspondence: Antonio Carlos Abramo, ACA - Institute of Assistance in Plastic Surgery, Rua Afonso de Freitas 641, São Paulo, SP, 04006-052, Brazil, Tel 55 11 3884.3671
Received: January 22, 2022 | Published: February 24, 2022
Citation: Abramo AC, Marcio SM. Nonsurgical rhinoplasty with hyaluronic acid to enhance precise areas of the nose and improve its overall shape and contour. J Dermat Cosmetol. 2022;6(1):9-13. DOI: 10.15406/jdc.2022.06.00199
Background: Nonsurgical Rhinoplasty (NSR) using soft-cohesive crosslinked hyaluronic acid (HA) was performed to reshape the nose appearance. Knowledge of the nasal anatomy mostly the blood supply, adequate placement of the HA, and potential complications became NSRHA safe and effective.
Method: Twenty females underwent NSR to enhance their nose appearance. Injection points were distributed along the nose midline from columella to the bridge. HA was injected supraperichondrial and supraperiosteally on columella, into the interdomal fat pad on tip, and into the deep fatty layer on dorsum and bridge. Bolus injection was made in the tip and columella. A single row retrograde flow injection was made in the dorsum-bridge line. ROE questionnaire assessed quantitatively the patient's self-consciousness of the nose appearance and the scores correlated with the images of the nose before- and after-NSR.
Results: NSR-HA was performed in a single session. Volume average per patient/treatment was 0.73 ml and per point/treatment was 0.17 ml. Comparing images of the nose before- and after-NSR showed a significant improvement of the nose appearance. Patient satisfaction was quantitatively demonstrated by the ROE questionnaire scores of 31.04% before-NSR against 73.33% and 70.00% at months one and twelve, respectively, after-NSR. The difference of 3.33%, greater at month one than month twelve, indicated no significant changes in the nose appearance for twelve months.
Conclusions: Positive evaluation of NSR-HA was evidenced comparing the nose images before, and at months one and twelve after HA injection validated by the patient self-consciousness of the nose appearance measured by the ROE questionnaire.
Keywords: nonsurgical rhinoplasty, filler, hyaluronic acid, nose appearance, nose reshaping, nasal envelope
Nonsurgical rhinoplasty (NSR) is an outpatient procedure ideal for individuals who are not ready for a permanent solution, downtime, or are worried about the risks involved in a traditional rhinoplasty. Hyaluronic acid (HA) derivatives are the most common injectables for NSR.1 Products differentiation is based on the rheologic property elastic modulus or G′ and physicochemical properties, as cohesivity.2 G' is crosslinking degree dependent, whereas cohesivity depends on HA concentration. The degree of crosslinking indicates the mechanical strength of the gel and represents a measure of HA durability.3 Long-lasting effects as 12 months or longer are reported to high cohesive soft-gel HA.4 Short-term side effects as bruising and erythema normally subside within days. Severe complications as cutaneous necrosis and blindness result from direct intravascular injection or compressive effect on local vessels.5 Appropriate knowledge of nasal vasculature, and choice of proper HA ensure safety and efficacy for NSR. As NSR-HA is associated with aesthetic outcomes, becomes relevant to understand the patient concept of self-image related to their nose appearance. Rhinoplasty Outcomes Evaluation (ROE) questionnaire assesses quantitatively patient's satisfaction and quality of life comparing its nose appearance before- and after-NSR.6 NSR using a soft-cohesive crosslinked HA is described here to reshape the nose. Quantitative validation of the images pre-and post-NSR, which indicate realistically achieved treatment outcomes, were correlated with the scores of the patient self-consciousness of the nose appearance before and after NSR-HA measured through the ROE questionnaire.
The nasal envelope consists of skin, two layers of fat, superficial musculoaponeurotic system (SMAS), perichondrium, and periosteum. The superficial fatty layer comprises subcutaneous tissue and the interdomal fat pad of the tip. Subcutaneous tissue is thin, tightly attached to the skin by fibrous septae, and supplied by major blood vessels arranged in a subdermal network.7 Interdomal fat pad is loose and thick composed only by adipocytes without fibrous septae and major blood vessels, with a structure differentiated from the fibroadipose tissue of the subcutaneous tissue.8 It occupies the space between the domes of the alar cartilages, with average dimensions of 2.8 x 4.1 x 13.7 mm.(Figure1) The deep fatty layer is wide and loose with a lack of fibrous septa and neurovascular structures.9 Between both fatty layers is the fibromuscular layer, a continuity of the facial SMAS. The blood supply of the external nose comes from the dorsal nasal branch of the ophthalmic artery, and the angular, lateral nasal, and superior labial branches of the facial artery. The dorsal artery supplies the dorsum, mostly at the osseous part, and the angular, lateral nasal and superior labial arteries supply the lateral aspect of the dorsum, tip, ala, and columella.(Figure 2) The arteries course in the fibromuscular layer, and mostly in the superficial fatty layer, creating several subdermal plexuses.10 The safe zone for injection is the nose midline from columella to bridge, supraperiosteal and supraperichondrial on the columella, into the interdomal fat pad of the tip, and the deep fatty layer of the dorsum and bridge. Danger zones are the fibromuscular layer and superficial fatty layer, mostly at the nasal root, sidewalls, ala, and tip (Figure 2).
Twenty females, aged from 39- to 49-year-old, mean age of 43.4-year-old, dissatisfied with the nose appearance, underwent NSR-HA. Exclusion criteria were males, smokers, bleeding disorders, allergy, nasal acne and trauma, scars, surgical procedures, and patients with nonrealistic outcome expectations. To prevent unequal diffusion of the gel, which could interfere with the results, all patients used the filler QTFill SIGNATURE SubQ, a high cohesive and high G' soft-HA with 24 mg/ml of HA crosslinked with BDDE 2.0 ppm and 0.3% lidocaine hydrochloride, from a single manufacturer (S. THEPHARM Co. Ltd., Seoul, Korea). Patients were followed for twelve months. All patients signed an informed consent form agreeing to the proposed treatment that was performed according to the 1964 Helsinki Declaration and Medical Research Involving Human Subjects.
Injection procedure
Topical anesthesia with lidocaine hydrochloride cream was used on the nose before injection. The nose was cleaned with alcoholic chlorhexidine solution before each injection. A 27G½ needle injected the HA slowly and carefully, observing the filling and color of the area, to avoid vascular disorders. Injection points were distributed along the midline of the nose, from the columella to the bridge, to prevent lateral deviation of the nose. HA was injected supraperiosteally at the base of the columella, supraperichondrial at the middle of the columella, into the interdomal fat pad of the tip, and deep fatty layer of the dorsum and bridge. Bolus injection was made in the tip and columella, whereas a single row retrograde flow injection was made in the dorsum and bridge. Pinching and lifting the skin in the tip, dorsum and bridge when injecting HA prevented major blood vessels compromise and lateral spread of the gel (Figure 3). Small volumes of HA per injection point were enough to remodel the nose.
Injection points
Columella - Point C1 was made over the nasal spine to increase the columella-labial angle (Figure 3A) and point C2 in the middle of the columella over the cephalic portion of the medial crus of both alar cartilages to project and elongate the columella (Figure 3B).
Tip - Point T1 was made between the domes of the alar cartilages in the caudal portion of the interdomal fat pad to project the tip forward (Figure 3C) and point T2 over the domes of the alar cartilages in the cephalic portion of the interdomal fat pad to project the tip upward (Figure 3D).
Dorsum - Point D was made at the caudal portion of the dorsum over the cartilaginous septum to thin, straighten and raise the dorsum (Figure 4A).
Nasal Bridge - Point NB was done at the upper half of the dorsum, over the bony hump to level the nasal bridge with the dorsum (Figure 4B).
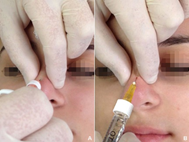
Figure 4 Pinching and lifting the skin throughout HA injection avoided to injure the major blood vessels of the nasal envelope (A) at the dorsum and (B) at the bridge.
Assessments
ROE questionnaire adjusted for NSR assess patient's self-consciousness of the nose appearance and quality of life before- and at months one and twelve after-NSR. The questionnaire is composed of six questions and measures the way patients perceive their nose appearance and the way they think the people around them view their nose appearance.11 The score of each question is divided by 24 and multiplied by 100, which leads to a score varying between 0 and 100, 0 indicating absolute disappointment of the patient and 100 absolute satisfaction. Patients with scores greater than 50% were considered satisfied.
Twenty females underwent NSR-HA in a single session on six nasal zones via twenty-four injection points on the columella, thirty-five on the tip, nineteen on dorsum, and six on the bridge, an average of 4.2 points per patient. Injection volume on the columella was 1.64 ml, 1.28 ml in point C1 and 0.36 ml in point C2; the tip received 4.06 ml, 0.91 ml in point T1 and 3.15 ml in point T2; the dorsum 8.63 ml, and the bridge 0.35 ml. The volume average per patient/treatment was 0.73 ml and per point/treatment 0.17 ml. Table 1 summarizes patient distribution and injection points, and volume of HA per patient/nasal zones. NSR-HA was an augmentation rhinoplasty, even though made the nose appear smaller by making it look thinner and straighter, with a pointed tip (Figure 5). The reshaped nose was featured by the increase of the columella-labial angle over 90 degrees and projection of the columella; pointer tip with elongation and projection rightly balanced anteriorly and upward, and a light point centered on its highest projection; and leveling, thinning, and straighten of the dorsum-bridge line (Fig.6). Stretching and straightening of the columella, and projection and thinning of the domes recovered the tip symmetry (Figure 7). Comparing the images of the patient before and after NSR-HA showed a significant aesthetic enhancement of the nose appearance, with the HA injected remaining in situ without evidence of significant resorption or loss of correction from month one to month twelve (Figure 8). Satisfaction and quality of life of the patient enhanced significantly with the improvement of the nose appearance after NSR-HA. The ROE questionnaire score of 31.04% before HA injection compared with the scores of 73.33% and 70.00% at months one and twelve after HA injection, validated quantitatively the noticeable improvement of the nose appearance after NSR-HA. Moreover, the score of 3.33% lesser in month twelve than in month one showed that there were no significant changes in the nose appearance for twelve months, endorsing the efficacy and long-lasting of NSR-HA. Table 2 summarizes the scores of the ROE questionnaire on patient self-consciousness of the nose appearance and quality of life. Adverse events as erythema, swelling, or bruising regressed spontaneously within days. No major complications as asymmetry, granuloma, skin necrosis, or blindness occurred in this series of patients.
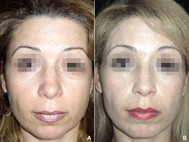
Figure 5 (A) A 49-year-old female with an enlarged dorsum and tip. (B) Improvement of the nose with 0.95 ml of HA distributed on six nasal zones.
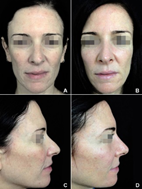
Figure 6 (A,C) A 48-year-old female with bridge enlargement and distorted dorsum, drop tip, and acute columella-labial angle. (B,D) A total of 0.88 ml of HA balanced and straightened the dorsum-bridge line, elongated and projected the tip, and projected the columella increasing the columella-labial angle.
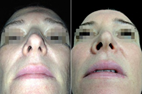
Figure 7 Basal view of the 48-year-old female. (A) Shortening of the columella and asymmetry of the tip. (B) A volume of 0.13 ml of HA increased the length of the columella, and 0.20 ml projected and refine the tip.
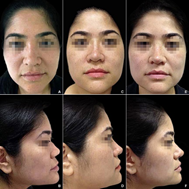
Figure 8 (A,B) A 39-year-old female with a hump, enlarged dorsum, drop tip and retracted columella. (C,D) A total of 0.94 ml of HA projected the columella, increased the columella-labial angle, pointed the tip, and projected and narrowed the dorsum-bridge line at one month after HA injection. (E,F) The reshaped nose remained stable with no significant changes until month twelve after HA injection .
age |
Columella |
tip |
dorsum |
nasal |
||||||||
C1 |
C2 |
T1 |
T2 |
bridge |
||||||||
1 |
42 |
0.05 |
0.03 |
0.07 |
0.13 |
0.4 |
- |
|||||
2 |
41 |
- |
- |
0.1 |
0.2 |
0.5 |
0.05 |
|||||
3 |
45 |
0,05 |
- |
0.05 |
0.12 |
0.53 |
0.05 |
|||||
4 |
39 |
0.1 |
0.05 |
0.05 |
0.15 |
0.45 |
- |
|||||
5 |
42 |
- |
- |
- |
0.1 |
0.5 |
- |
|||||
6 |
39 |
0.1 |
0.03 |
0.05 |
0.15 |
0.5 |
- |
|||||
7 |
49 |
0.1 |
0.05 |
0.05 |
0.2 |
0.5 |
0.05 |
|||||
8 |
43 |
- |
- |
0.07 |
0.4 |
- |
- |
|||||
9 |
43 |
0.1 |
0.03 |
- |
0.15 |
0.37 |
0.05 |
|||||
10 |
45 |
0.1 |
- |
0.08 |
0.12 |
0.55 |
- |
|||||
11 |
46 |
- |
- |
- |
0.1 |
0.35 |
- |
|||||
12 |
42 |
0.1 |
0.04 |
0.08 |
0.17 |
0.5 |
- |
|||||
13 |
46 |
0,05 |
- |
0.05 |
0.15 |
0.4 |
- |
|||||
14 |
45 |
0,08 |
- |
- |
0.12 |
0.58 |
- |
|||||
15 |
46 |
0.1 |
0.05 |
- |
0.17 |
0.33 |
- |
|||||
16 |
48 |
0.1 |
0.03 |
0.08 |
0.12 |
0.45 |
0.1 |
|||||
17 |
41 |
- |
- |
0.05 |
0.15 |
0.4 |
- |
|||||
18 |
41 |
0,05 |
- |
0.03 |
0.13 |
0.5 |
- |
|||||
19 |
46 |
0.1 |
- |
0.05 |
0.15 |
0.3 |
- |
|||||
20 |
39 |
0.1 |
0.05 |
0.05 |
0.17 |
0.52 |
0.05 |
|||||
Table 1 Patient distribution. Injection points and volume of HA for nasal zones
Questions |
Answers |
||
pre NSR |
01 month |
12 months |
|
after NSR |
after NSR |
||
|
|
|
|
1. How well do you like the appearance of your nose?* |
0.55±0.14 |
3.65±0.33 |
3.30±0.25 |
2. How well are you able to breathe through your nose?* |
2.90±0.25 |
2.85±0.23 |
2.80±0.25 |
3. How much do you feel your friends and loved ones like your nose?* |
1.05±0.11 |
3.40±0.38 |
3.30±0.44 |
4. Do you think your current nasal appearance limits your social or professional activities?** |
1.25±0.21 |
3.60±0.36 |
3.50±0.35 |
5. How confident are you that your nasal appearance is the best it can be?* |
0.80±0.10 |
3.55±0.36 |
3.45±0.36 |
6. Would you like alter the appearance of your nose nonsurgically with hyaluronic acid?*** |
0.90±0.67 |
0.55±0.09 |
0.45±0.06 |
Table 2 ROE questionnaire to assess the patient self-consciousness of its nose appearance
*Answer ranged from 0 (not at all) to 4 (completely) **Answer ranged from 0 (Always) to 4 (Never)
** Answer ranged from 0 (definitely) to 4 (no)
Nonsurgical rhinoplasty with fillers was an useful, temporary, minimally invasive alternative to conventional rhinoplasty. Various soft-tissue fillers, as autologous graft and HA, among others, appeared to be similarly effective and safe within a period, however, the ease of obtaining, storing, and cost guided the use of HA in NSR.12 To acquire more accurate outcomes, NSR-HA involved only females, since nasal dimensions and skin thickness were larger in males than females.13,14 The 39-49 age-group was chosen to be the middle-age, once the tip resistance became less intense the older the patient's age-group.15 According to Ahn et al.,16s needles and cannulas of the same gauge showed different inner and outer diameters. As the amount of the filler injected was determined by the inner diameter, only needles were used to prevent dispersion of the gel outside of the injection site. Crosslinked HA fillers with high G' and high cohesivity have large resistance to stretch and flattening with high durability in the injection site, properties required for a nasal filler.17 Despite its high cohesivity and elasticity, the gel of the filler QTFill SIGNATURE SubQ exhibited softness, smooth flow, and mostly a compact and homogeneous tissue diffusion, which rendered it more stable into the nasal envelop, without significant changes in the nose appearance for twelve months. As HA degradation occurred on the external surface of the gel and inside it, the small volume injected, and compactness of the gel delayed the enzymatic acting, ensuring long-lasting of the NSR-HA.18 Small volumes of HA, from 0.45- to 0.95-ml per patient, were sufficient for a pronounced effect in remodeling the nose. Overcorrection was avoided to prevent a delayed distorting augmentation of the nose, due to the hygroscopic property of the HA fillers to absorb water, mostly in the first month after application.19 The width of the dorsum and nasal bridge was effectively controlled with the HA injected in a single row than multiple rows. HA injected on the nose midline without deviating from it minimized the possibility of nasal asymmetry.20 The most important anatomical considerations in NSR-HA were the vascular network and its relationship with the fat layers, of great relevance to avoid potential risk of intravascular injections, or compressive effect on local vessels. Injections over the bone and cartilage at the columella, into the interdomal fat pad of the tip, and into the deep fat layer of the dorsum and bridge created a safe protocol for NSR-HA, since the nasal arteries coursed in the musculoaponeurotic system and subcutaneous tissue.21 Unlike Humphrey et al., 22 HA was not injected at the root, ala, and mostly at sidewalls, once the gel could pressure the subdermal nasal vasculature, with risk of tissue necrosis. Efficacy and long-lasting of the NSR-HA were clinically and quantitatively well-evidenced. No significant changes in the nose images from month one to month twelve showed that the HA injected remained stable without evidence of significant resorption or loss of correction for twelve months. Quantitative validation was established by the scores of 31.04% before- and 73.33% at month one and 70.00% at month twelve after-NSR. The scores showed a significant positive correlation between the increase of patient satisfaction and quality of life, with clinical improvement of the nose appearance after NSR-HA.
None.
The author declares there is no conflict of interest.

©2022 Abramo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.