Journal of
eISSN: 2574-9943


Case Report Volume 5 Issue 1
University of Queensland, Australia
Correspondence: David John Mackay Smith, University of Queensland, Australia
Received: January 14, 2021 | Published: January 27, 2021
Citation: Smith DJM. Metastatic melanoma presenting as a malignant peripheral nerve sheath tumour. J Dermat Cosmetol . 2021;5(1):1-4. DOI: 10.15406/jdc.2021.05.00172
This is a report of a metastatic melanoma presenting clinically as a soft tissue mass and histologically being diagnosed as a malignant peripheral nerve sheath tumour. In this case the metastatic melanoma was preceded by a primary cutaneous melanoma in a similar anatomical region.
Histologically the tumour was characterised by a malignant-appearing Spindle cell proliferation, arranged in fascicules. There was no evidence of connection to a nerve, co-existent neurofibroma or stigmata of neurofibromatosis.
This presentation is only infrequently mentioned in the literature and heterogeneity can make clinical and histological diagnosis of metastatic melanoma problematic. It can easily be misinterpreted without effective clinico-histological correlation, making a good working relationship between Clinician and Histopathologist essential for correct diagnosis.
A 58 year old male patient appeared at a dedicated skin cancer clinic in a regional centre for general skin check.
There was no specific history of melanoma or nonmelanoma skin cancer, although there was a family history of melanoma.
On examination, there was found to be a small pigmented papule present on the lower back. The lesion rested on a pale elliptical patch that appeared to represent a previous skin excision. On interrogation the patient was evasive and initially denied any knowledge of an excision. At a subsequent consultation he admitted to having an excision in Thailand 2 years previously but failed to have any follow-up.
The lesion was a pigmented, 3mm diameter, raised papule with well demarcated border. It had the superficial appearance and colouration of a haemangioma but was structureless, lacking lacunae. The lesion was biopsied.
Histology gave a diagnosis of Nodular melanoma, Breslow 0.65mm, Clark level III. There was no ulceration and a mitotic rate of 1/mm2.
The patient reappeared a week later for wider excision. A small non-pigmented fibrous mass adjacent to the original scar was also biopsied (Figure 1).
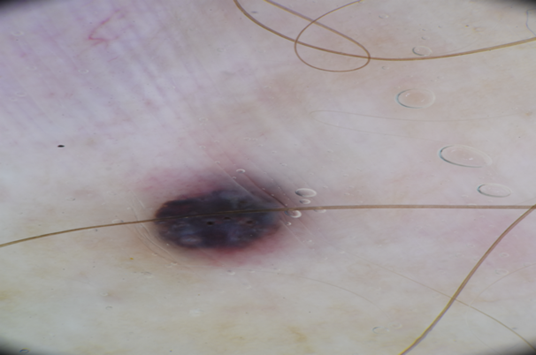
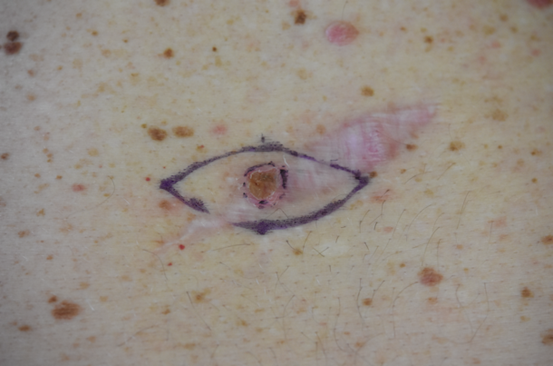
Figure 1 Upper image: Dermoscopic view of initial pigmented lesion.
Lower image: preparation for wider excision with metastatic lesion visible at the top of the image.
At histology, no residual melanoma was seen with the wider excision but the other lesion revealed a spindle cell nodule with fascicular pattern, Beslow 4.0mm, 4mitoses/mm2 and no epidermal component. S100 positive, CD10 negative.
The patient was referred to a Melanoma unit in the capital city. On assessment, it was felt that he only required further wide excision and was referred back to the regional public hospital. He had 2 further wider excisions, right inguinal node resection and eventually put on Keytruda and followed up at an oncology outpatient’s clinic.
At his last Oncology review, 22 months after the initial diagnosis, he was found to have a soft subcutaneous mass in close proximity to the original lesion. This was felt to be benign and he was referred back to the skin clinic doctor who performed the initial biopsy.
The lesion was excised and found to be a well circumscribed soft non-pigmented mass, 20X25X20mm, in approximately the same anatomic area as the original biopsy.
Initial histological report: A well delineated nodule of spindle-shaped neoplastic cells forming a fascicular pattern with areas of myxomatous degeneration and up to 5mitoses/mm2. The lesion was highly vascular. Provisional diagnosis of an atypical spindle cell lesion, Immunoperoxidase stains pending. Additional report: Negative for Sox10, AE1/3 and SMA. Focally positive for CD34. Preliminary impression: “It is unlikely to be a metastatic melanoma. A neural or nerve sheath differentiation is favoured (Figure 2).”
At this stage there was a discussion between histologist and clinician with the suggestion to refer back to the initial slides and re-examine this patient’ history.
A final report was issued and immunostaining repeated, additionally Desmin, EMA and C-kit were also negative. “Only a few scattered cells are positive for Sox 10. Focal positive staining is noted around some intra-tumoural blood vessels for S100 and CD34. CD34 is usually a haemopoietic cell marker but examples of melanoma with aberrant expression of this marker can be found in the literature. Dedifferentiated melanoma can mimic many other malignancies in H&E sections. N.B. Previous reports have been noted. Diagnosis: consistent with metastatic nodular melanoma. “
In embryonic development, peripheral nervous tissue and melanocytes arise from a common neuro-ectodermal source as part of the neural crest. The progenitors are multipotent and are capable of self-renewal and as adult cells some retain stem cell-like characteristics.
Neural crest cells (NCC) represent an important transient cell population in early development of vertebrates. They delaminate from the dorsal neuroepithelium and migrate away to generate a variety of cell types. In the trunk, early cells migrate ventrally providing glia, sympathetic and sensory neurons, later cells adopting a melanocytic fate. Melanocytes also migrate in a dorsolateral pathway later but begin to differentiate earlier as melanoblasts (Figure 3 ). As part of the glia, Schwann cell precursors (SCPs) retain their plasticity and multipotent characteristics.1
Studies of zebrafish mutant pigment patterns showed that proliferative pigment cell precursors are associated with peripheral nerves and ganglia, and migrate to the hypodermis where they differentiate as melanophores. This helped explain the mechanism of many phenotypes and the roles for latent precursors in adult homeostasis, regeneration and neoplasia.2
The main cell types of NCCs migrating in the ventral migration pathway include sensory and sympathetic neurons and SCP cell types. The SCPs are the cellular source of Schwann cells, melanocytes and endothelial fibroblasts.
Adameyko et al were able to demonstrate SCP-to-melanocyte transition in vivo with mouse and chick embryos in normal development. They identified growing nerves projecting throughout the body as a stem/progenitor niche containing SCPs from which large numbers of skin melanocytes originate (figure 4).
On dorso-lateral migration, NCCs adopt a melanoblast fate shortly after delamination, mainly proliferating at their final destination. With ventral migration, NCCs associated with nerves acquire a SCP fate and migrate along both dorsal and ventral rami. At the ends of nerves some SCPs acquire a melanocyte fate, detach from nerves and proliferate within the skin. (Adapted from Ernfors 2010).
They also found that Schwann cell and melanocyte development share signaling molecules with both the glial and melanocyte cell fates linked to nerve contact and regulated in an opposing manner by various growth factors.3 All melanocytes are specified by microphthalmia-related transcription factor (MITF), that activates many genes required for melanogenesis. They found a peripheral decline in Sox2 in nerves might make SCPs susceptible for an induction of MITF and differentiation into melanocytes4 (figure 4).
In the development of melanocytes and neurons the two cell types follow similar migration pathways to the periphery and their mature structure, consisting of extended dendritic processes to communicate with surrounding cells is also similar. In both cell types their differentiated function is influenced by neighboring cells. The central and peripheral nervous systems share the same signaling molecules with melanocytes. (Table 1).
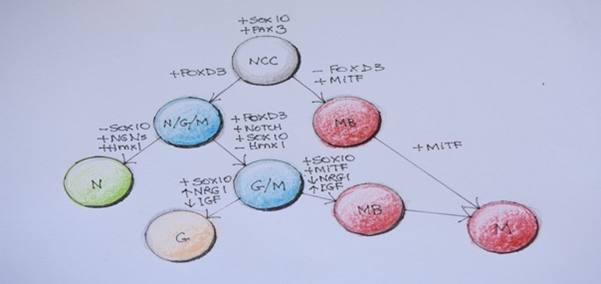
Figure 5Transcription factors and hierarchical relationships associated with diversification of Neural crest cells in the trunk.
NCC, neural crest cells; N/G/M, neuronal/glial/melanoblast progenitors; MB, melanoblast;
NRG1, neurogenin 1; IGF, insulin-like growth factor; Hmx 1-homeobox transcription factor; N, neuronal committed cell; G-glial cells; M-melanocyte. (Adapted from Ernfors 2010)
Signal molecule |
receptor |
phase |
melanocyte |
neurone |
Wnt |
Frizzled |
Development |
MITF transcription |
Differentiation |
BMP |
BMP receptor |
Development |
Anti-differentiation |
Anti-differentiation |
Endothelin 1 |
Ednr A |
adult |
DNA repair |
survival |
Endothelin 3 |
Ednr B |
Development |
migration |
Migration anti-differentiation |
SCF |
c-Kit |
Development/ adult |
Migration |
Differentiation |
FGF |
FGF receptor |
Development/ |
Mitogen |
Proliferation differentiation |
HGF |
Met |
Development /adult |
Proliferation |
Differentiation survival |
NGF |
p75NTR/ |
Development/ adult |
Survival |
Survival cognition |
Neurotrophin 3 |
p75NTR/ |
Development |
Survival |
Survival |
Brain-derived neurotrophic factor |
p75NTR/ |
Development |
|
Survival |
Neurotrophin |
p75NTR/ |
Development |
|
Survival |
semaphorins |
β1integin |
Development |
dendricity |
Synapse |
Table 1 Signaling molecules affecting both melanocytes and neurones
Adameyko et al concluded that a large number of melanocytes in hair follicles and the interfollicular dermis, representing nearly all populations of melanocytes in the trunk skin of adult mice, are of SCP origin. They also showed that myelinating mature Schwann cells retain the capacity to differentiate into melanocytes after injury.3 Nerve innervation is required for tissue regeneration,5 most cells that mediate skin tissue repair are derived from cutaneous nerves, upregulating Sox2 after injury6 and the requirement of Schwann cells from injured nerves as part of the wound healing process.7
SCPs are implicated in several kinds of tumours with melanocytic and neuronal features;
Since melanocytes and Schwann cells can originate from SCPs, such associations usually complicates the diagnosis of these tumours, expressing both melanocytic and Schwann cell markers.
There is no doubt about the malignant and metastatic potential of melanoma tumorigenesis, and the heterogeneity and plasticity of their cells. This is in part related to their ability to hijack embryonic developmental programs to achieve pleuripotency and mobility, particularly in relation to recapitulation of epidermal-mesenchymal transition. After injury or tumiorgenic processes mature Schwann cells can get reprogrammed and acquire multipotent properties characteristic of SCPs.
An interesting study by Iyengar showed that melanoma cells could express characteristics of glia on interaction with endothelial cells when looking at patterns of neuronal differentiation in relation to angiogenesis in vertical growth phase melanoma. His results showed that melanoma cells have the potential for differentiation into glial as well as neuronal cells. The formation of a structured perivascular mantle zone during tumour-vascular interactions recapitulating embryonic neurogenesis. Melanoma cells acting as neuronal stem cells.8
Bidirectional cellular communication is integral to cancer progression and embryonic development. Aggressive tumor cells are phenotypically plastic, sharing properties with embryonic progenitors. In development, multipotent precursor cells are specified to fates through autocrine and paracrine signaling molecules. There is a similar progression of melanoma with melanoma cells that release and receive cues that promote proliferation and metastases without the inhibition of tumour suppressor elements present in development.
There are no conflicts of interest.
None.
None.

©2021 Smith. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.