Journal of
eISSN: 2574-9943


Research Article Volume 8 Issue 3
1Medical School of ABC, Department of Dermatology, Brazil
2Department of ultrasound of University of São Paulo (USP), Brazil
3Mato Grosso Federal University (UFMT), Brazil
Correspondence: Luciana Gasques, Faculdade de Medicina do ABC, Santo André, SP, Brazil, Tel +5565999393366
Received: July 06, 2024 | Published: August 15, 2024
Citation: Gonzaga M, Bruno PA, Sigrist R, et al. Improvement of skin laxity and the lifting effect with the use of poly-l-lactic acid Rennova Elleva®. J Dermat Cosmetol. 2024;8(3):67-72. DOI: 10.15406/jdc.2024.08.00269
Poly-L-lactic acid (PLLA) is a biocompatible, absorbable, immunologically inert polymer that induces neocollagenesis. In the late 1990s, it began to be used as a bio-stimulator not only for the treatment of skin aging and other causes of facial lipoatrophy, but also as cosmetic indications in non-facial areas to improve skin quality. About two years ago, a new presentation of poly-L-lactic acid was launched in Brazil under the name RennovaElleva®.
The product RennovaElleva® has been approved by ANVISA for the treatment of skin laxity, leading to the restoration of facial volume and tissue repositioning on the face and body. Each vial contains 210 mg of poly-L-lactic acid (PLLA), carboxymethylcellulose (132 mg) and mannitol (178 mg). It comes in the form of a lyophilized powder that should be reconstituted in 16 ml of final diluent volume.
In this article, we describe the results obtained using the vector application technique, which aimed at improving skin laxity and facial contour with a lifting effect in ten female patients, with two sessions spaced 45 days apart. In addition to the clinical results achieved at 3 and 6 months after the end of treatment, safety and efficacy outcomes were analyzed over this period. We also discuss the distinctive characteristics of this new presentation.
Keywords: poly-L-lactic acid, bio-stimulators, facial rejuvenation, lifting
The aging process in the face begins gradually, around the age of 30, when the rate of cell renewal starts to slow down.1 However, the manifestations may take years to become noticeable and are characterized by the thinning of the dermal and hypodermal layers, skin atrophy, soft tissue descent, and loss of bone tissue.2,3
Understanding the anatomy of aging has led to a significant shift in facial treatment, establishing the three-dimensional concept that recognizes volumetric losses and facial fat redistribution as signs of aging.1 This concept is based on balancing various facial structures, taking sex, ethnicity, and each patient's objectives into account, resulting in more natural and harmonious outcomes in treatment. Minimally invasive techniques for facial rejuvenation involve the use of fillers, volumizers, and bio-stimulators,4,5 either individually or simultaneously, and the improvement of skin laxity has become the primary objective in many treatments.
Although no standardization has been established so far, the vector technique has been shown to yield excellent results in repositioning facial structures and attenuating effects that occur as a consequence of the facial aging process. It has been described both for use with hyaluronic acid-based fillers and with bio-stimulators such as poly-L-lactic acid and calcium hydroxyapatite. As a matter of fact, the use of poly-L-lactic acid (PLLA) has grown exponentially.6–8
Poly-L-lactic acid (PLLA) is a biocompatible and biodegradable synthetic polymer derived from lactic acid, belonging to the family of alpha-hydroxy acids. Its mechanism of action consists of stimulating subclinical tissue inflammation, attracting fibroblasts, which results in fibroplasia, encapsulation of microparticles, and deposition of type III collagen, followed by its remodeling into type I collagen in the extracellular matrix.8,9 This fibroplasia achieves the desired cosmetic outcome through a controlled inflammatory reaction. New collagen begins to form approximately one month after treatment and continues to increase over a period of nine months to one year after each application. At Month 6, many particles become porous and are surrounded by macrophages. The product degrades through non-enzymatic hydrolysis into lactic acid monomers, which are metabolized into CO2, H2O, or incorporated into glucose. After 9 to 12 months, there is no evidence of fibrosis, and PLLA particles disappear. The half-life of PLLA is estimated to be 31 days, and it is completely eliminated from the body in approximately 18 months.9
PLLA has demonstrated excellent outcomes as a bio-stimulator that promotes progressive and prolonged improvement in skin quality and laxity, resulting in enhanced facial contour and tissue repositioning. However, a consensus regarding the most suitable facial areas or those offering the best outcomes for this treatment has not yet been reached. Therefore, we aimed to establish a vector technique, describing treatment areas that yield the most favorable outcomes in repositioning facial tissues, promoting a more defined contour and lifting effect with natural and harmonious aesthetic effects for patients with skin laxity.
This study was conducted at Marisa Gonzaga da Cunha Dermatologic private clinic and approved by the ethics committee of FMABC. It adhered to all ethical standards and guidelines established in the Helsinki Declaration (world Medical Association, 2013). The protocol amendments and informed consent forms were approved by the Ethics Committee. We evaluated the clinical response of treated patients using various methods: digital photography, ultrasound documentation and patient-completed questionnaires. Safety, durability, and adverse events were also analyzed.
The product used in the treatment was RennovaElleva®, approved by ANVISA under number 80451960236 as a Medical Device Risk classification IV. It is presented as lyophilized powder in a sterile amber vial containing non-pyrogenic mannitol, sodium carboxymethylcellulose as an emulsifier, and 210 mg of PLLA in the form of microparticles with a narrow particle-size distribution and more uniform sizes. Its lyophilization technology allows for reconstitution within a maximum of 1 hour external to the vial.10
Inclusion criteria: Healthy, omnivorous women with mild to moderate facial laxity, according to the Facial Laxity Rating Scale Validation Study.11 Ages between 30 and 48 years, stable weight, no comorbidities, and no prior treatments with bio-stimulators or fillers.
Exclusion criteria: Women with severe facial laxity, pregnancy and lactation, use of corticosteroids and immunosuppressants, smoking, diabetes, hypertension, and restrictive dietary regimens for weight loss.
Patients were instructed to maintain stable weight and dietary habits throughout the treatment and evaluation period, and to refrain from other facial treatments, such as technologies and fillers. Topical treatment with moisturizers and sunscreen was maintained.
Evaluations: Patients were informed about the importance of photographic documentation, as the procedure would be performed with gradual benefits over approximately 6 months. Evaluations were conducted using digital photographs taken from frontal and lateral (45-degree and 90-degree) angles at the following time points: 0 (before the first application), 1 (45 days after the first application and before the second application), 2 (90 days after the second application), and 3 (180 days after the second application). The camera used was Sony Lens G Cyber-shot 16.1 mega pixels (DSC-H90) with automatic correction. standardized object distance of 100cm, without zoom.
The skin ultrasound examination was performed using high-resolution ultrasound devices LOGIQ e® and LOGIQ E10 (GE Healthcare, Waukesha, WI), equipped with high-frequency linear transducers of 10-22 MHz and 6-24 MHz, during the pre-treatment period, 90 days, and 180 days after the second application. The aim was to demonstrate the positioning and distribution of RennovaElleva® in the tissues, as well as its longevity and distribution over the 6-month period post-application. Four points on the face were marked, and 5 measurements of the epidermis-dermis complex and hypodermis were taken around each marked point (Figure 1).
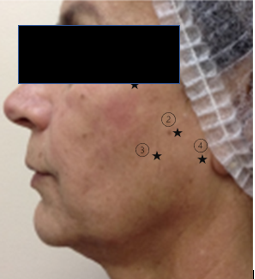
Figure 1 Measurement points for the ultrasound examination. Five measurements were taken around each point.
A questionnaire was also administered during periods 0 to 3 to assess the outcomes from the patients' perspective, as well as the presence of any adverse and collateral effects. At period 0, the initial degree of skin laxity, the presence and depth of nasolabial fold (NLF), marionette lines, and malar depression were evaluated. The score varied between 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). In subsequent periods, improvement in skin laxity, nasolabial fold, marionette lines, and malar region was assessed. Patients rated the changes as 0 (no improvement), 1 (slight improvement), 2 (moderate improvement), and 3 (significant improvement). Satisfaction with the treatment was rated on a scale from 0 to 10 based on the improvement in skin quality and the lifting effect achieved.
Product preparation
The reconstitution of RennovaElleva® was performed one hour before its use with 16 ml of distilled water for injection, as per the package insert instructions.
Applications
The treatment areas were marked according to Figures 1A–1G, which were consistent for all patients. The application was performed under anesthesia with 2% lidocaine at the cannula entry points after rigorous skin antisepsis with chlorhexidine. Cold compresses were applied before and after injections to reduce pain and promote vasoconstriction, aiming to minimize bruising and hematoma formation. The injections were administered using 3 ml syringes and 22G/50mm cannulas.
The cannula entry points were created with a 21G needle angled at 30-45 degrees to the skin at the following locations: jawline angle, pretragal region, above the zygomatic arch, and malar region (4 points on each side). Isolated linear retroinjection (tracts) or the fanning techniques were used, depending on the treatment area, delivering 8 ml of RennovaElleva on each side of the face into the superficial hypodermis. The product was distributed as follows: along the side of the face from the jawline angle (3 ml – divided into 5 tracts of 0.6 ml – Figure 1A) and the tragus to the corner of the mouth (1 ml in retroinjection with 0.5 ml tracts – Figure 1B), using retroligamentary applications; hypodermis over the zygomatic bone in small boluses of 0.16 ml at 3 points (total 0.5 ml – Figure 1C); temporal region (1 ml – divided into 0.3 ml tracts after aspiration – Figure 1D); retro temporal region (1 ml divided into 0.3 ml tracts after aspiration – Figure 1E); and malar region (in isolated 0.5 ml tracts – Figure 1F). After facial applications, the remaining product (2 ml) was diluted in an equal part of distilled water and injected in four linear retroinjections of 0.5 ml into the submental region bilaterally, distributed in a fan-shaped pattern as shown in the attached diagram (Figure 1G). The applications were consistently performed in the same sequence: from lower to upper parts and from lateral to medial regions of the face.
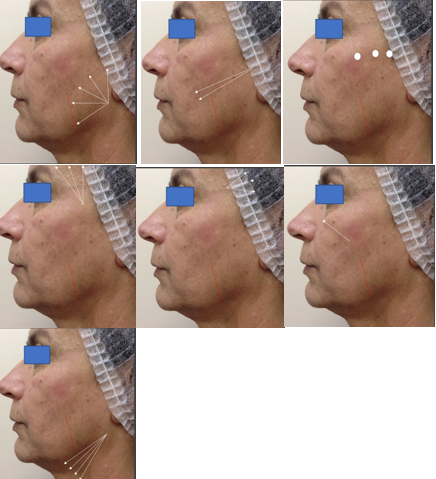
Figures 1A – 1G Cannula entry points and application in linear retroinjections with isolated or fan-shaped distribution.
To prevent superficial deposits, injections were stopped when ¾ of the needle was visible. The applications, with few entry punctures, were carried out in a continuous motion during retroinjection, in a fan-like pattern, through several retrograde tunnels to cover larger areas and achieve optimal product spread. The syringe was kept parallel to the skin surface during application to keep the cannula patent throughout the procedure. The treated area was immediately massaged to ensure even distribution of the product. Two sessions were performed with a 45-day interval between them.
After each treatment, the patient was instructed to massage the area five times a day for five minutes, using emollient creams to minimize friction during massage.11 This massage helps distribute the product and aids in preventing the formation of papules and nodules.5
Ten women aged between 32 and 48 years (mean age 39 years) presented with mild skin laxity (1 patient) and moderate skin laxity (9 patients); mild nasolabial fold (5 patients), moderate nasolabial fold (5 patients), and severe nasolabial fold (1 patient); absent marionette lines (3 patient), moderate marionette lines (5 patients), and severe marionette lines (2 patient); malar region with absent depression (2 patients), mild depression (2 patients), and severe depression (6 patients) (Table 1).
Score of involvement – pre-application score
|
Patient |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
Age (years) |
48 |
32 |
44 |
38 |
33 |
36 |
49 |
44 |
35 |
38 |
|
Laxity |
2 |
1 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Nasolabial fold (NLF) |
1 |
1 |
2 |
1 |
1 |
1 |
3 |
2 |
2 |
2 |
|
Marionette lines |
1 |
0 |
0 |
1 |
1 |
1 |
2 |
2 |
1 |
0 |
|
Malar depression |
1 |
0 |
0 |
1 |
2 |
2 |
2 |
2 |
2 |
2 |
Table 1 Score of involvement for skin laxity, nasolabial fold (NLF), marionette lines and malar depression
Regarding the results, evaluations were conducted at 3 and 6 months after the second application based on responses from a questionnaire. The scores were as follows: moderate improvement in skin laxity (4 patients) and significant improvement in skin laxity (6 patients); moderate improvement in nasolabial folds (6 patients) and significant improvement (4 patients); moderate improvement in marionette lines (4 patients) and significant improvement (5 patients). As for satisfaction level: rating of 7 (1 patient), rating of 9 (2 patients), and rating of 10 (7 patients) (Table 2).
Improvement rating – scores at 6 months after the second application
|
Patient |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
Age (years) |
48 |
32 |
44 |
38 |
33 |
35 |
49 |
44 |
35 |
38 |
|
Firmness |
2 |
3 |
2 |
3 |
2 |
3 |
3 |
2 |
3 |
3 |
|
NLF |
2 |
3 |
2 |
2 |
2 |
3 |
2 |
2 |
3 |
3 |
|
Marionette lines |
2 |
0 |
2 |
3 |
3 |
3 |
2 |
3 |
3 |
3 |
|
Malar depression |
2 |
0 |
0 |
2 |
3 |
3 |
2 |
2 |
3 |
3 |
|
Satisfaction rating |
7 |
10 |
10 |
9 |
9 |
10 |
10 |
10 |
10 |
10 |
Table 2 Results of scores 6 months after the second application. The improvement rating and satisfaction level were consistent in both assessments
Regarding adverse effects: mild pain (5 patients) to moderate pain (5 patients) after the first application, and mild pain (7 patients) and moderaste pain (3 patients) after the second application; discreet bruises at the puncture sites in 2 patients after the second application. Small non-visible palpable nodules (less than 1 cm) appeared in the left malar region (2 patients) and unilateral zygomatic arch (2 patients), which resolved without treatment over time and were absent at the second assessment.
Digital photographic evaluation
The lifting effect was observed immediately after the first application (Figures 2A–2C). However, that was a transient result that lasted 1-2 days and was related to the volume of the reconstituted product.
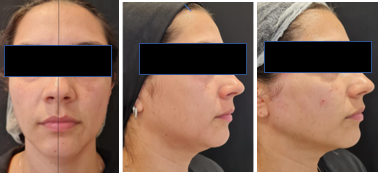
Figure 2A Patient 6 (36 years old). Digital photos - Right side treated and left side untreated immediately after the first application, demonstrating immediate tissue repositioning and the achieved lifting effect.
Figure 2B Pre-treatment.
Figure 2C Immediately after treatment.
Evaluation of the results focused on improvement in skin laxity, reduction of wrinkles, and enhancement of facial contour. Photos were taken at pre-application, 3 months, and 6 months after the second application (Figures 3–5).
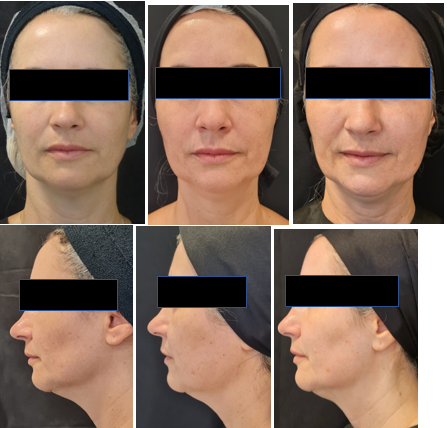
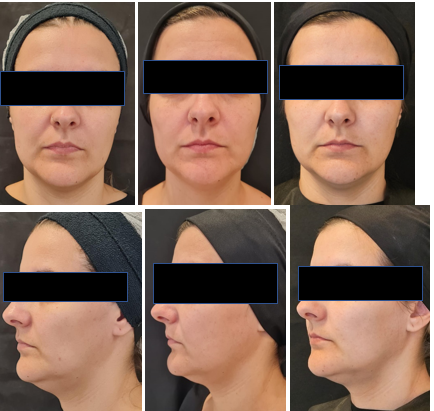
Figures 3 Patient 1 (48 years old) with a lean and elongated face. Digital photos from the front and in profile at 90 degrees. Photos were taken at pre-application, 3 months, and 6 months after the second application, showing improvement in contour, periorbital region, submental laxity, and wrinkles. The patient reported a satisfaction rating of 7 with the treatment.
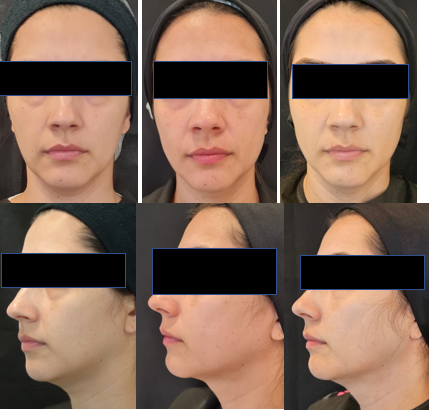
Figures 4 Patient 6 (36 years old) with an oblique face. Digital photos from the front and in profile at 45 degrees. Photos were taken at pre-application, 3 months, and 6 months after the second application, demonstrating improvement in contour and wrinkles. The patient also noted improvement in the periorbital region, although it was not the focus of the study. The satisfaction rating was 10.
Figures 5 Patient 9 (35 years old) with an elongated face. Digital photos from the front and in profile at 45 degrees. Photos were taken at pre-application, 3 months, and 6 months after the second application, showing improvement in contour, submental laxity, and wrinkles. The patient also reported improvement in the periorbital region. The satisfaction rating was 10.
During the ultrasound examination, the average of the 5 measurements obtained at the 4 assessment points in the pre-treatment periods, 90 and 180 days after the second application in millimeters are described in Table 3.
|
Period |
0 |
0 |
2 |
2 |
3 |
3 |
|
|
Pacient |
Age |
Dermis |
Hipodermis |
Dermis |
Hipodermis |
Dermis |
Hipodermis |
|
1 |
48 |
0,135 |
0,307 |
0,154 |
0,368 |
0,155 |
0,384 |
|
2 |
32 |
0,162 |
0,204 |
0,196 |
0,234 |
0,195 |
0,281 |
|
3 |
44 |
0,140 |
0,425 |
0,138 |
0,372 (*) |
0,138 |
0,471 |
|
4 |
38 |
0,129 |
0,345 |
0,135 |
0,404 |
0,140 |
0,511 |
|
5 |
33 |
0,137 |
0,217 |
0,137 |
0,288 |
0,146 |
0,307 |
|
6 |
35 |
0,139 |
0,375 |
0,138 |
0,454 |
0,171 |
0,431 |
|
7 |
49 |
0,154 |
0,270 |
0,151 |
0,297 |
||
|
8 |
44 |
0,107 |
0,365 |
0,125 |
0,391 |
0,128 |
0,448 |
|
9 |
35 |
0,169 |
0,369 |
0,144 |
0,411 |
0,175 |
0,437 |
|
10 |
38 |
0,177 |
0,501 |
0,158 |
0,456 (*) |
0,180 |
0,531 |
Table 3 Measurements in millimeters of the dermis and hypodermis in the ultrasound examination in periods 0 (pre-application), 2 (90 days after the second application) and 3 (180 days after the second application), demonstrating the increase in hypodermis measurements mainly. Patients with (*) had lost weight during this period
In addition to measurements of the epidermis-dermis complex and hypodermis, the ultrasonographic characteristics of PLLA were evaluated. At 90 and 180 days, all patients exhibited solid nodular structures in the superficial hypodermis, mostly non-palpable, hypoechoic, with well-defined contours, which, on color Doppler study, showed slight vascularity (Figure 7).
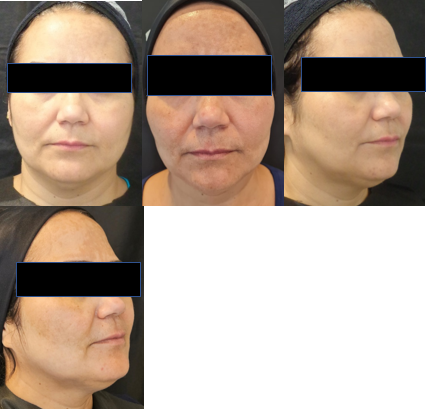
Figure 6 Patient 3 (44 years old) with rounded face. Pre-application photos and 6 months after the second application, with improvement in the contour and submental sagging.
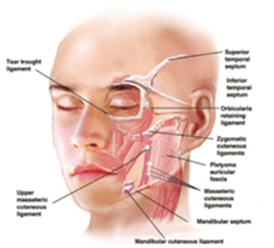
Figure 7 Distribution of facial ligaments.14
Figure 7 High-resolution ultrasound with a 24 MHz linear transducer demonstrating nodulariform (*) structure in the superficial hypodermis, 90 days after PLLA application.
Although PLLA (Poly-L-lactic acid) has been used for facial rejuvenation and volumization for several years, consensus and standardization of applications have not yet been established. Consequently, disastrous hard-to-treat outcomes have been observed.
Treatment with PLLA promotes the formation of a new collagen matrix in response to the deposition of the microparticles, increasing tissue volume6 with a consequent improvement of skin laxity8 without necessarily volumizing the face. Therefore, for younger patients who may not yet have significant volume loss but show signs of skin laxity due to factors such as body type, genetics, and diet, PLLA treatment becomes an optimal therapeutic option.
In the photographic analysis, an immediate lifting effect can be seen after the first application. However, this effect is transient, lasting 1-2 days and is due to the reconstituted volume of the product.
Image analysis after 3 and 6 months demonstrated improvement in contour, periorbital region, submentor sagging and wrinkles. The camera used was Sony Lens G Cyber-shot 16.1 mega pixels (DSC-H90) with automatic correction. Standardized object distance of 100cm, without zoom.
Thus, the main objective of this study was to establish standardized application techniques to achieve a lifting effect with harmonious and natural results, evaluating outcomes over 6 months after 2 applications. The focus was on the repositioning of facial structures without increasing facial volume.
The applications were strategically distributed across different areas of the face to achieve overall facial volume repositioning (lifting effect), with emphasis on the improvement of skin quality in the treated areas. The location of treatment areas considered the distribution of ligaments and their insertion into the dermis (Figure 7).7,12–14
Applications in the retro ligamentous spaces and just below dermal insertion points promoted the lifting effect by safely and naturally repositioning adipose pads. The use of 3 ml syringes made handling more comfortable and allowed for their manipulation to prevent PLLA precipitation and needle or cannula obstruction.
The interval between sessions recommended in the literature for PLLA can range between 4 and 8 weeks, and the number of sessions varies until the desired results are achieved.15 Therefore, we chose 45-day intervals between applications. Rendon, in a five-year retrospective follow-up study, suggests that results depend on patient age, initial dermal thickness, and pre-treatment bone structure,15 indicating that patients with better bone contour show greater lifting effects.
In our study, we observed that patients over 44 years old, with potentially lower collagen and elastin production, still showed significant improvement in skin quality (Table 2). However, there was less effect on reducing nasolabial folds and marionette lines, consistent with the observation that older patients may require more applications or vials to achieve desired results.
Photographic analysis, an immediate lifting effect can be seen after the first application. However, this effect is transient, lasting 1-2 days and is due to the reconstituted volume of the product. image analysis after 3 and 6 months demonstrated improvement in contour, periorbital region, submentor sagging and wrinkles.
Several studies demonstrate patient satisfaction rates following treatment. Vleggaar reported a 95.1% satisfaction rate among patients with PLLA treatment.8 In the current study, the high level of patient satisfaction with treatment (Table 2) reveals that the technique used achieved the desired results of improving skin laxity and lifting effect, using few product vials and avoiding unwanted facial volumization.
Adverse reactions observed with PLLA use are primarily related to injection sites, such as bruising, hematomas, edema, papules, nodules, and granulomas. Papules and nodules are mostly palpable rather than visible and depend on the application technique.16–18 They are associated with large volumes injected superficially,19 failure to pause injection before withdrawing the needle,20 the use of minimally diluted product,10 injections into thin skin areas like the infraorbital, perioral and temporal regions17 and hypermobile areas,20 intradermal injections,21 and lack of post-procedural massage.21 Intervals of 4-6 weeks between sessions minimize nodule formation.21
Regarding the ultrasonographic characteristics of the product, a change was observed in the intrinsic pattern of PLLA, previously described by the authors Cunha MG,22 as tiny focal hyperechoic deposits at the time of application. After 90 days post-application, there was the appearance of hypoechoic nodular structures, which may correspond to a tissue reaction or the presence of deposited material that had not been absorbed by the evaluated period. Histopathological study could clarify the nature of these structures; however, since these structures were non-palpable and non-visible, and due to patients' unwillingness to undergo examination, this possibility was discarded.
In the present study, pain and hematoma formations were attributed to the application technique rather than the product itself. Regarding the formation of nodules, two patients noted the appearance of small, palpable, non-visible nodules unilaterally in the malar region and two in the unilateral zygomatic arch after the first application, accounting for 4.76% of the total treated points (Figures 1A–1G). These nodules were most likely related to the mobility of the treated area and did not require treatment since they spontaneously reduced in size over time. This data reveals that the superficial hypodermic technique used, with a more dispersed distribution of PLLA particles, minimizes the occurrence of common adverse effects such as nodule formation. Additionally, the product RennovaElleva® exhibits a more uniform tissue distribution of particles, probably due to its controlled particle size.`22 The recommended reconstitution of 16 ml in distilled water proved to be safe with few early or late adverse effects.
Poly-L-lactic acid has been used for over two decades to improve skin texture and volumize the face. Its use is safe and effective for correcting unesthetic scars and treating skin laxity, providing predictable and durable results. With the correct dilution and application technique using vectors of RennovaElleva®, it was possible to achieve a safe and long-lasting natural lifting effect without unnecessary facial volumization or significant adverse effects. An evaluation over 12 months will be valuable to assess the durability of results and the behavior of the observed deposits.
None.
The authors declare no conflict of interest.
None.

©2024 Gonzaga, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.