Journal of
eISSN: 2574-9943


Review Article Volume 9 Issue 3
1Department of Pharmacy, Yogendra Nath Saxena College of Pharmacy & Research Centre, Amroha, Uttar Pradesh, India
2Department of Pharmaceutical Chemistry, R V Institute of Pharmacy, Bijnor, Uttar Pradesh, India
3Department of Pharmacology, Indubhai Patel College of Pharmacy & Research Centre, Anand, Gujarat, India
4Department of Pharmacology, Metro College of Health Sciences & Research, Greater Noida, Uttar Pradesh, India
Correspondence: Gosiya, Department of Pharmacy,Yogendra Nath Saxena College of Pharmacy & Research Centre, Amroha, 244241, Uttar Pradesh, India, Tel +91-8439475882
Received: July 03, 2025 | Published: July 18, 2025
Citation: Gosiya, Giri N, Rouman A, et al. Hyaluronic acid in modern Cosmeceuticals: a review of skin health and anti-aging innovations. J Dermat Cosmetol. 2025;9(3):60-69. DOI: 10.15406/jdc.2025.09.00293
Hyaluronic acid (HA), a naturally occurring glycosaminoglycan (GAG), has emerged as a pivotal bioactive ingredient in modern cosmeceuticals due to its remarkable biocompatibility, hydrophilicity, and multifunctional dermatological benefits. This review explores the structural, pharmacological, and therapeutic dimensions of HA, particularly in the context of skin hydration, repair, and anti-aging. HA's molecular weight (MW) plays a critical role in its bioactivity. High molecular weight (HMW) HA offers surface-level hydration and anti-inflammatory effects, while low MW-HA penetrates deeper to promote collagen synthesis and tissue regeneration. Topical formulations, injectable dermal fillers, and advanced delivery systems such as nanoparticles and microneedles have broadened HA’s clinical utility and consumer appeal. Mechanistically, HA interacts with receptors such as cluster of differentiation 44 (CD44) and the receptor for hyaluronic acid-mediated motility (RHAMM), influencing the functions of keratinocytes and fibroblasts, while also regulating immune responses through Toll-like receptors 2 and 4 (TLR2/TLR4). These receptor-mediated pathways enhance skin elasticity, accelerate wound healing, and mitigate oxidative stress. Recent advances include cross-linked and bioengineered HA derivatives with improved stability and prolonged activity. Moreover, combination therapies incorporating HA with peptides, retinoids, or vitamins exhibit synergistic benefits in anti-aging and scar treatments. Safety assessments affirm HA’s excellent tolerability, reinforcing its dominant role in both cosmetic and therapeutic dermatology. Emerging frontiers in personalized HA-based skincare and its potential role in regulating the skin microbiome signal forward-looking strategies for innovation. Overall, HA continues to redefine cosmeceutical science by offering multifunctional, evidence-based solutions for skin health and rejuvenation.
Keywords: anti-aging, extracellular matrix, collagen synthesis, Cosmeceuticals, dermal fillers, nanoparticles, transdermal delivery, artificial intelligence
HA, hyaluronic acid; GAG, glycosaminoglycan; MW, molecular weight; HMW, high molecular weight; CD 44, cluster of differentiation 44; RHAMM, receptor for hyaluronic acid-mediated motility; TLR2/TLR4, toll-like receptors 2 and 4; ECM, extracellular matrix; TEWL, trans-epidermal water loss; LMW, low molecular weight; AI, artificial intelligence
The global cosmeceutical industry has undergone a significant transformation in recent years, driven by growing consumer demand for scientifically backed, multifunctional skincare products.1 Cosmeceutical products that lie at the fusion of cosmetics and pharmaceuticals offer both aesthetic and therapeutic benefits. Among the many active ingredients used in modern formulations,2 hyaluronic acid, also known as Hyaluronan, has emerged as one of the most prominent and widely researched biomolecules.3,4 Originally discovered in the 1930s, HA was initially valued for its unique viscoelastic and hydrophilic properties, but it is now recognized for its broad applicability in dermatology, particularly in the areas of skin hydration, repair, and anti-aging interventions.5,6 The rise of HA in cosmeceutical formulations is rooted in both its biological relevance and its exceptional pharmacological profile.7 As a naturally occurring GAG found in the extracellular matrix (ECM) of human connective tissue, skin, and synovial fluid, HA plays a vital role in maintaining skin structure, moisture balance, and cellular signaling.8 The concentration of HA in the dermis decreases significantly with age and environmental stressors, contributing to common signs of skin aging such as dryness, loss of elasticity,9 and the formation of fine lines and wrinkles.10 This physiological decline has led to the widespread incorporation of HA in both topical and injectable skincare products aimed at restoring hydration and youthful appearance.11 In the context of dermato-pharmacology, HA is of particular interest due to its dual function as both a passive moisturizer and an active modulator of skin physiology. Its high biocompatibility, non-immunogenic nature, and ability to interact with specific cell surface receptors such as CD44 and RHAMM make it an ideal candidate for therapeutic use.12 Furthermore, advancements in formulation technology, ranging from nanocarriers and cross-linked gels to multi-molecular weight systems, have enhanced HA's bioavailability and penetration into different skin layers, thereby expanding its clinical efficacy, market, and consumer demand.13 These innovations have positioned HA not only as a cosmetic agent but also as a key pharmacological tool in managing skin aging and related dermatological conditions.14 Given its multifunctional roles, increasing clinical validation, and consumer preference for effective, science-driven skincare, HA continues to redefine the landscape of modern cosmeceuticals.16 This study aims to provide a comprehensive analysis of the structural, pharmacological, and therapeutic dimensions of HA, with a focus on its application in skin health, anti-aging strategies, and recent innovations in delivery technologies.
Chemical structure and physicochemical properties
HA is a linear polysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine, linked by alternating β-1,4 and β-1,3 glycosidic bonds.17 Its strong hydrophilicity, viscoelastic nature, and MW-dependent bioactivity contribute to its multifunctional role in skin hydration, elasticity, and anti-aging therapy.18
Molecular structure
HA is a naturally occurring, unbranched linear polysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine.19 These monomers are alternately linked by β-1,3 and β-1,4 glycosidic bonds, forming the backbone structure of the polymer. The general repeating unit can be denoted as [→4) β-D-GlcA (1→3) β-D-GlcNAc (1→]ₙ,20 where GlcA refers to glucuronic acid and GlcNAc to N-acetylglucosamine (Figure 1).21 Unlike other GAGs, HA is non-sulfated and not covalently bound to a protein core, which distinguishes it structurally and functionally.22 The presence of multiple hydroxyl, carboxyl, and acetamido functional groups imparts strong hydrophilicity and enables HA to form a highly hydrated, gel-like matrix within the extracellular space. This molecular configuration plays a critical role in the maintenance of skin hydration, viscoelasticity, and tissue homeostasis, and serves as the basis for its significant use in topical, injectable, and transdermal cosmeceutical formulations.23
Hydrophilicity and viscoelasticity
HA is highly hydrophilic due to the presence of multiple hydroxyl, carboxyl, and acetamido groups capable of forming strong hydrogen bonds with water molecules. This characteristic allows it to absorb and retain large amounts of water up to 1000 times its weight, making it an ideal natural moisturizing agent in dermatology and cosmetology.26 Its viscoelastic properties are another defining physicochemical feature, enabling HA to behave as a viscous fluid under low shear conditions and as an elastic solid under high shear stress.27 These rheological behaviors depend on concentration, MW, and polymer entanglement. In synovial fluid, for example, these properties contribute to shock absorption and lubrication, while in skin care, they help in enhancing texture and application feel, and maintaining skin tone.28
Molecular weight implications
The biological and pharmacological activities of HA are strongly influenced by its MW, which can range from 5 kDa to over 10,000 kDa. Based on MW,29 HA can be categorized into three types:
High molecular weight (>1000 kDa)
Exhibits anti-inflammatory and immunosuppressive effects. It forms a protective film on the skin surface and prevents trans-epidermal water loss (TEWL), making it ideal for surface hydration and skin barrier reinforcement in topical applications.30
Medium molecular weight (~250-1000 kDa)
Provides moderate skin penetration and hydration. It balances the properties of HMW and low molecular weight (LMW), often used in formulations targeting both surface and mid-layer skin hydration.31
Low molecular weight (<250 kDa)
Penetrates deeper into the epidermis, potentially stimulating cell proliferation, collagen synthesis, and wound healing. However, extremely LMW (<50 kDa) may occasionally exhibit pro-inflammatory effects, necessitating careful optimization in product formulations.32 The ability to engineer HA at specific MW or combine multiple weights in one formulation has allowed the development of multifunctional cosmeceuticals that hydrate, protect, and biologically stimulate the skin at multiple levels.33
Pharmacokinetics and pharmacodynamics
HA, though primarily known for its topical and injectable use in dermatological and cosmetic medicine, exhibits a complex pharmacokinetic and pharmacodynamic profile that underpins its biological effectiveness in cosmeceuticals and therapeutic applications.34
Absorption, distribution, metabolism, and excretion
HA is absorbed based on its route of administration, with topical forms acting locally and injectables reaching deeper tissues. It is distributed in connective tissues, is metabolized by hyaluronidases (HYAL1 and HYAL2), and is excreted via the lymphatic system and kidneys (Figure 2).35,36
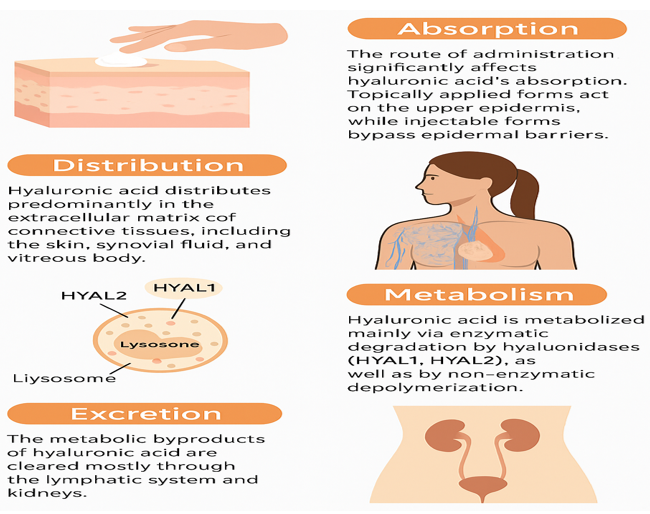
Figure 2 Pharmacokinetic pathway of HA (HA’s absorption (topical/injectable), distribution in connective tissues, enzymatic metabolism by HYAL1 & HYAL2, and excretion via the lymphatic system and kidneys).
Absorption
The route of administration significantly affects HA’s absorption. Topically applied HA, especially HMW forms, primarily exerts effects on the stratum corneum and upper layers of the epidermis due to its large size and limited skin permeability. However, recent advances in LMW-HA formulations, nano-encapsulation, and chemical modifications have improved dermal penetration and enhanced systemic and localized effects. Injectable forms, such as dermal fillers or intra-articular injections, bypass epidermal barriers and are directly delivered to target tissues.37
Distribution
Once absorbed or injected, HA distributes predominantly in the ECM of connective tissues, including the skin, synovial fluid, and vitreous body. Its distribution is dependent on its MW; smaller fragments are more readily distributed systemically, whereas larger polymers remain localized at the injection site and serve as a scaffold or hydrating reservoir.38
Metabolism
HA is metabolized primarily through enzymatic degradation by hyaluronidases (HYAL1 and HYAL2), which break the glycosidic bonds between the disaccharide units. HYAL2, located on the cell surface, initiates HA breakdown into intermediate-size fragments, which are subsequently internalized and further degraded in lysosomes by HYAL1. In tissues, oxidative stress and reactive oxygen species can also contribute to non-enzymatic depolymerization of HA.39
Excretion
The metabolic byproducts of HA are cleared predominantly via the lymphatic system and kidneys. Small oligosaccharides resulting from degradation are excreted in the urine.40 The systemic half-life of HA is relatively short, ranging from minutes to a few hours, depending on molecular size and route of administration. In contrast, cross-linked HA (used in dermal fillers) may persist in tissues for several months due to its resistance to enzymatic breakdown.41
Cluster of Differentiation 44
A widely expressed transmembrane glycoprotein involved in cell adhesion, migration, proliferation, and inflammation. Binding of HA to CD44 influences keratinocyte function, fibroblast activity, and immune modulation, making it central to skin repair and anti-aging effects.42
Receptor for hyaluronic acid-mediated motility
This receptor is associated with cytoskeletal remodeling, cell motility, and wound healing. HA-RHAMM interactions promote fibroblast migration and tissue regeneration, contributing to HA’s effectiveness in rejuvenation therapies.43
Toll-like receptors 2/4
LMW-HA fragments may act as danger-associated molecular patterns and bind to TLRs, regulating immune responses and influencing inflammation. This dual role is size-dependent: while HMW-HA is anti-inflammatory, LMW-HA can be pro-inflammatory under certain conditions.44
Mechanism of action in skin physiology
HA hydrates the skin by retaining water, stimulates collagen synthesis via fibroblast activation, and supports epidermal barrier repair through enhanced keratinocyte function. It also exhibits antioxidant and anti-inflammatory effects, protecting skin from oxidative stress and promoting regeneration (Figure 3).45
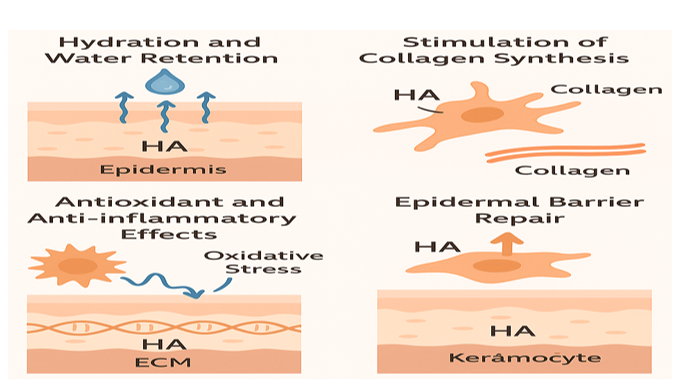
Figure 3 HA’s role in skin health: promoting hydration, enhancing collagen synthesis, providing antioxidant and anti-inflammatory effects, and supporting epidermal barrier repair.
Hydration and water retention
HA plays a vital role in maintaining skin hydration due to its unique ability to bind and retain up to a thousand times its weight in water.46 This hydrophilic nature allows HA to form a viscoelastic, gel-like matrix in the extracellular space, enhancing skin tone and hydration level, smoothness, and elasticity. It acts as a reservoir for moisture, particularly in the stratum corneum and dermis, maintaining optimal hydration levels essential for barrier integrity and cell viability.47
Stimulation of collagen synthesis
HA contributes indirectly to collagen production by creating a hydrated environment that supports fibroblast function and ECM remodeling. HA-CD44 interactions stimulate fibroblasts to synthesize type I and III collagen, crucial for skin firmness and elasticity. This regenerative property is further amplified by cross-linked HA used in cosmetic procedures, which prolongs fibroblast activation and dermal matrix reinforcement.48
Antioxidant and anti-inflammatory effects
HMW-HA exhibits antioxidant activity by scavenging reactive oxygen species, thereby protecting skin cells from oxidative stress and UV-induced damage.49 It also suppresses inflammatory pathways by down regulating pro-inflammatory cytokines and inhibiting the activity of enzymes like matrix metalloproteinases, which degrade collagen. Conversely, LMW-HA fragments may stimulate a transient inflammatory response beneficial for wound healing and tissue renewal.50
Epidermal barrier repair
HA aids in restoring the epidermal barrier by promoting keratinocyte proliferation, migration, and differentiation. It strengthens cell-cell junctions and enhances the expression of tight junction proteins,51 leading to improved barrier function and reduced TEWL. HA also accelerates re-epithelialization and facilitates the repair of microdamage, making it a key agent in formulations aimed at treating dry, irritated, or aging skin.52
Formulation strategies in Cosmeceuticals
Cosmeceutical formulation strategies encompass a range of innovative delivery systems designed to optimize the efficacy of active ingredients. Topical formulations such as creams, gels, and serums are widely employed to provide hydration and deliver targeted skin benefits.53 Injectable formulations, notably dermal fillers composed of HA, offer long-lasting effects in restoring volume and reducing the appearance of wrinkles. Advanced delivery technologies, including liposomes, nanoparticles, and microneedles, enhance the penetration and bioavailability of active ingredients,54 overcoming the barrier function of the stratum corneum.55 The MW of compounds, particularly HA, plays a pivotal role in determining their cutaneous absorption, biological activity, and depth of action within the skin layers.56
Topical delivery systems
Creams, gels, and serums are among the most widely used vehicles in cosmeceutical formulations. Creams and lotions, typically emulsions of oil and water, provide excellent moisturization and are ideal for delivering HMW ingredients like HA that act on the skin surface to retain moisture and protect the barrier.57 Gels, on the other hand, are non-greasy and suitable for oily or sensitive skin types, allowing better incorporation of low to medium MW active ingredients. Serums are lightweight and fast-absorbing aqueous formulations designed for deeper penetration of active ingredients like peptides, antioxidants, and LMW-HA. Their minimal base and high concentration of active ingredients make them highly effective for targeted skin benefits such as hydration, brightening, and anti-aging.58
Injectable formulations
The dermal fillers based on HA have gained popularity in cosmetic dermatology. These formulations often consist of cross-linked HA, which provides viscoelasticity and volumizing effects when injected into the dermis or subcutaneous tissue.59 The degree of cross-linking and particle size determine the filler’s viscosity, longevity, and suitability for different facial areas. These injectables can last from six months to over a year, offering temporary but significant improvement in facial wrinkles, volume loss, and contouring.60 The minimally invasive nature and immediate results make dermal fillers a preferred choice for non-surgical facial rejuvenation.61
Advanced delivery methods
Liposomes, nanoparticles, and microneedles are revolutionizing the effectiveness of cosmeceuticals by enhancing skin penetration and targeted delivery of active ingredients. Liposomes, which are phospholipid vesicles, encapsulate both hydrophilic and lipophilic actives and merge with skin membranes to release contents effectively.62 Nanoparticles, including solid lipid nanoparticles and nanostructured lipid carriers, offer controlled release, improved stability, and better penetration of active ingredients into deeper skin layers. Microneedles are another cutting-edge technology that creates microchannels in the skin, facilitating the transdermal delivery of macromolecules like peptides, growth factors, and even HMW-HA, which would otherwise have limited permeability through the stratum corneum.63
Role of molecular weight (Low vs High) in efficacy
The MW of active ingredients, especially HA, plays a crucial role in determining their efficacy and depth of action. HMW-HA (>1000 kDa) remains on the skin surface, forming a hydrating and protective film, reducing TEWL, and providing anti-inflammatory effects. In contrast, LMW-HA (<100 kDa) penetrates deeper into the epidermis and dermis, where it can interact with cellular receptors such as CD44 and stimulate biological responses like collagen synthesis, tissue repair, and skin rejuvenation. Therefore, a combination of different MWs in a single formulation can offer both immediate surface hydration and long-term dermal benefits.64
Therapeutic and cosmetic applications of hyaluronic acid
HA plays a pivotal role in both therapeutic and cosmetic dermatology due to its exceptional hydrating, regenerative, and biocompatible properties (Figure 4).65 It enhances skin hydration and elasticity by retaining moisture in the epidermis, thereby improving suppleness and reducing dryness. HA-based dermal fillers are widely used to minimize fine lines and wrinkles by restoring volume and stimulating collagen synthesis.66 Additionally, HA promotes wound healing and scar reduction by controlling inflammation and supporting tissue repair. In acne and photo damage management, HA soothes irritated skin, reduces inflammation, and aids in skin regeneration, making it an essential ingredient in anti-aging and regenerative skincare regimens (Table 1).67–69
|
S. No. |
Benefit |
Mechanism of action |
Representative examples |
|
1 |
Skin hydration and elasticity |
HA binds and retains large amounts of water in the epidermis, maintaining moisture and improving skin hydration and elasticity |
Topical HA serums (Neutrogena Hydro Boost), HA-enriched moisturizers, Injectable HA fillers (Juvederm Volite) |
|
2 |
Reduction of fine lines and wrinkles |
HA’s hydrating and volumizing effects plump the skin, smoothing wrinkles and fine lines. Injectable fillers restore volume loss |
Dermal fillers like Juvederm, Restylane; HA serums used in anti-aging skincare |
|
3 |
Wound healing and skin repair |
HA modulates inflammation, promotes cell migration and proliferation, facilitating faster tissue repair. |
Topical wound healing gels and creams containing HA |
|
4 |
Improvement in skin barrier function |
HA supports epidermal barrier integrity by maintaining hydration and supporting keratinocyte function |
Moisturizers with HA that improve barrier function, reducing TEWL |
|
5 |
Anti-inflammatory effects |
HA interacts with cell receptors (CD44), controlling inflammatory responses in the skin |
Therapeutic topical formulations for sensitive or inflamed skin |
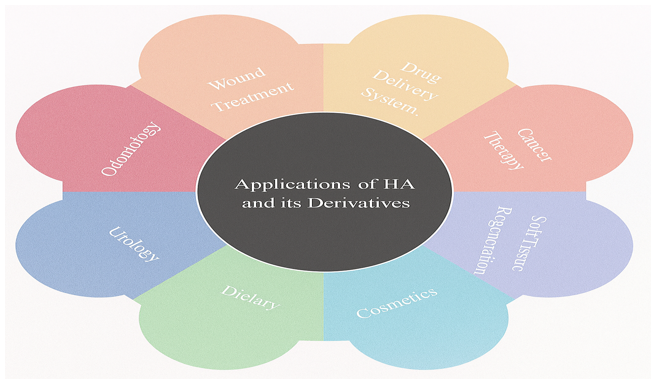
Figure 4 Broad-spectrum applications of HA and its derivatives across various medical and therapeutic domains.
Skin hydration and elasticity improvement
HA’s high water-binding capacity helps retain moisture in the epidermis, improving skin tone and elasticity. It reinforces the skin’s natural barrier, leading to increased elasticity and a rejuvenated appearance. Topical formulations and injectable HA (dermal fillers) are commonly used for deep moisturization and volumizing effects.70
Reduction of fine lines and wrinkles
HA-based dermal fillers (such as non-crosslinked or crosslinked HA) are widely used in aesthetic dermatology to fill wrinkles and restore lost facial volume. HA smoothens the skin surface by attracting and holding water in the dermis, providing an immediate lifting effect and long-term structural support through stimulation of collagen production.71
Wound healing and scar treatment
HA modulates inflammation, promotes fibroblast migration, and supports re-epithelialization. These properties accelerate tissue regeneration and healing. HA-containing dressings and gels are used for treating chronic wounds, burns, and surgical scars by maintaining a moist environment and reducing scar formation.72
Acne and photodamage management
In acne treatment, HA hydrates and soothes irritated skin, reduces post-inflammatory hyperpigmentation, and enhances skin repair. For photodamaged skin, HA improves texture and tone by restoring moisture, reducing oxidative stress, and assisting in dermal remodeling. It is often combined with antioxidants or retinoids for synergistic benefits.73
Innovations and recent advances
Recent innovations and advances in cosmeceutical science have significantly enhanced the functionality and versatility of HA-based products. One of the breakthroughs is the development of cross-linked HA technologies, which involve chemically modifying HA to form a stable 3D network.74 This cross-linking improves the viscoelastic properties, resistance to enzymatic degradation, and durability of HA in dermal filler applications. In addition, the advent of bioengineered HA and synthetic derivatives has enabled the production of highly pure, tailored MW-HA with enhanced biocompatibility and targeted biological activity. These novel forms provide better control over hydration, anti-aging effects, and tissue regeneration. Furthermore, combination therapies incorporating HA with bioactive molecules such as peptides, vitamins (vitamin C and E), and growth factors have shown synergistic effects, promoting collagen synthesis, cellular repair, and skin rejuvenation.75 These multi-functional formulations address multiple signs of aging and skin damage more effectively than HA alone. Complementing these innovations are smart delivery systems and sustained-release platforms, including hydrogel matrices, nanocarriers, and pH or temperature-responsive vehicles. These systems ensure controlled and prolonged delivery of HA and other active ingredients, minimizing dosing frequency while maximizing therapeutic outcomes and patient compliance.76
Safety, tolerability, and adverse effects
HA is widely regarded as a safe and biocompatible substance in both clinical and cosmetic applications due to its natural presence in the human body. Clinical data have consistently shown that HA-based topical and injectable products exhibit excellent tolerability, with minimal risk of skin irritation, sensitization, or allergic reactions. Most adverse events are mild and temporary, such as localized erythema, swelling, or tenderness at the injection site in dermal filler applications. Allergic responses are rare, particularly with non-animal stabilized HA preparations.77
Biocompatibility studies indicate that HA does not typically induce immunogenic responses and is generally well-accepted by skin and connective tissues. High-purity, bioengineered forms of HA further minimize the risk of contamination and endotoxin-related reactions. For long-term use, data support the safety of HA in repeated applications, although monitoring is advised in sensitive individuals or those with a history of dermal allergies.78
From a regulatory perspective, HA-based products are subject to oversight by agencies such as the United States Food and Drug Administration (U.S. FDA), European Medicines Agency (EMA), and Central Drugs Standard Control Organization in India. Topical HA formulations are typically classified as cosmetics or over-the-counter products, requiring only basic safety evaluations. In contrast, injectable HA fillers are regulated as medical devices (Class III in the U.S.) or combination products and must meet safety, efficacy, and manufacturing standards, including preclinical and clinical trial data submission.79 Overall, HA remains one of the most biocompatible and clinically validated ingredients in dermatology and cosmetic medicine, with a favorable safety profile across diverse formulation types and usage scenarios. Nonetheless, adherence to good manufacturing practices, patient screening, and post-market surveillance is crucial to maintaining safety and public trust in HA-based products.80
Comparative efficacy with other Cosmeceutical agents
HA holds a unique position among cosmeceutical ingredients due to its exceptional water-binding capacity, viscoelastic properties, and compatibility with various skin types. However, when compared to other widely used agents like collagen, retinoids, peptides, and Niacinamide, the distinct mechanisms and benefits of each compound underscore the value of both individual and combined use in advanced skincare formulations.81
Collagen vs hyaluronic acid
Both collagen and HA are naturally occurring components of the ECM and play vital roles in skin structure and hydration. While collagen provides structural support and mechanical strength, it has poor skin penetration due to its HMW when applied topically.82 HA, on the other hand, particularly in LMW forms, penetrates deeper into the skin and significantly improves hydration, skin firmness, and elasticity. Injectable collagen has become less favored due to allergenicity risks, while HA-based dermal fillers have largely replaced it because of superior safety and prolonged effectiveness.83
Retinoids vs hyaluronic acid
Retinoids, including retinol and tretinoin, are vitamin A derivatives known for promoting cellular regeneration, reducing wrinkles, and treating acne. However, they can cause dryness, irritation, and photosensitivity. HA serves as an excellent complementary agent by counteracting the dehydration and irritation commonly associated with retinoid use. While retinoids actively remodel the skin at a cellular level, HA passively supports this process by maintaining moisture and barrier integrity.84
Peptides vs hyaluronic acid
Peptides, especially signal peptides and carrier peptides, stimulate collagen synthesis, enhance skin firmness, and support wound healing. Their action complements HA's hydrating function by promoting structural protein regeneration. When used in combination, HA ensures an optimal microenvironment for peptide function by maintaining hydration and facilitating deeper skin penetration.85
Niacinamide vs hyaluronic acid
Niacinamide (Vitamin B3) is known for its anti-inflammatory, brightening, and barrier-repair properties. It regulates sebum production, improves pigmentation, and reduces redness. Unlike HA, which hydrates passively, niacinamide actively enhances the skin barrier and reduces TEWL. Combined use of niacinamide and HA provides comprehensive skin benefits: hydration from HA and barrier repair, plus anti-aging effects from niacinamide.86
Synergistic effects and combination use
Recent formulation trends focus on synergistic blends of HA with these cosmeceuticals to target multiple skin concerns. For instance, HA-retinoid serums are increasingly popular for anti-aging regimens, as HA offsets the irritation of retinoids while maintaining hydration.87 Similarly, HA-peptide serums promote skin strength and hydration simultaneously, while HA-niacinamide formulations balance moisture retention with anti-inflammatory benefits. These combinations not only enhance the efficacy of each active but also improve user tolerability and compliance.88
Market trends and consumer perspectives
The global demand for HA in cosmeceuticals is rising rapidly, driven by its anti-aging and hydrating benefits. Consumers increasingly prefer scientifically backed, transparent formulations, but challenges remain in standardized labeling and substantiated marketing claims.89
Global hyaluronic acid cosmeceutical market growth
The global cosmeceutical market, particularly products featuring HA, has experienced exponential growth in recent years. As of the latest market estimates, the global HA-based skincare market is valued at over USD 9 billion, with projections suggesting it will surpass USD 15 billion by 2030, driven by increased demand for anti-aging, hydrating, and minimally invasive aesthetic solutions.90,91 The rise in the aging population, greater awareness of skin health, and the growing popularity of non-surgical cosmetic procedures have contributed significantly to this expansion. HA is a flagship ingredient in serums, moisturizers, facial masks, and injectable dermal fillers, making it a key driver in the cosmeceutical and aesthetic dermatology industries across North America, Europe, and the Asia-Pacific region (Figure 5).92–94
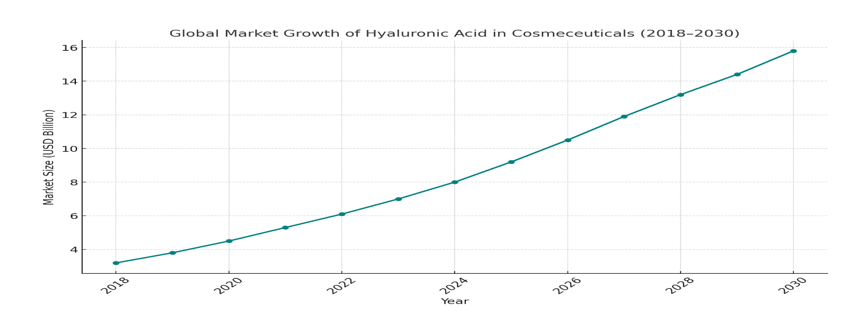
Figure 5 The global market growth of HA in Cosmeceuticals (2018 to 2030). The trend highlights a gradual increase in market size, projected to reach approximately USD 15.8 billion by 2030.
Consumer preferences and formulation transparency
Modern consumers are increasingly educated and selective about the ingredients in their skincare products. There is a strong preference for clean, scientifically backed, and sustainably sourced ingredients. HA fits this profile, especially when labeled as non-animal derived or bio-fermented, which appeals to vegan and cruelty-free markets. Consumers also value the MW of HA, often seeking products with a blend of HMW and LMW-HA for both surface hydration and deeper penetration.95
Formulation transparency has become a priority. Shoppers demand clear ingredient lists, knowledge about product sourcing, and evidence-based efficacy claims. Brands that disclose the type of HA used, concentration, and clinical benefits gain higher consumer trust. Additionally, the demand for minimalist, multifunctional formulations has led to the rise of combination products where HA is paired with peptides, vitamins, or plant-based antioxidants for comprehensive skin benefits.96
Challenges in product labeling and claims
Despite its popularity, the cosmeceutical market faces challenges related to regulatory standards, labeling, and marketing claims. In many countries, including the U.S. and India, cosmeceuticals fall into a regulatory gray area not classified as either pure cosmetics or drugs, allowing room for ambiguous or exaggerated marketing claims.97 Products often label HA-containing formulations as clinically proven or anti-aging, without substantiating these claims with robust clinical trials.98
Furthermore, the lack of standardization in labeling HA types (sodium hyaluronate vs hydrolyzed HA vs cross-linked HA) creates confusion among consumers and limits informed decision-making. Regulatory agencies like the U.S. FDA and EMA do not require pre-approval for cosmetic formulations, which sometimes leads to misleading representations of product efficacy, MW impact, or HA concentration.99 To address these challenges, there is a growing push for harmonized regulations, clinical validation of claims, and greater industry accountability in HA cosmeceutical labeling and promotion.100
Future directions in research and development
The future of HA in cosmeceuticals is marked by exciting advancements. Next-generation HA derivatives, such as thiolated or PEGylated HA, are being designed for enhanced stability, targeted delivery, and prolonged skin effects. Personalized cosmeceuticals are emerging, where artificial intelligence (AI) driven diagnostics and genetic profiling allow formulation of HA products tailored to individual skin types and needs.101 Additionally, HA is being investigated for its role in regulating the skin microbiome, offering potential for formulations that support skin health through microbiota balance. These innovations will shape more effective, science-driven, and consumer-responsive HA-based skincare.102
Next-generation HA derivatives
The future of HA research lies in the development of next-generation derivatives that offer enhanced bioactivity, stability, and targeted performance. Scientists are engineering HA molecules through chemical modifications such as sulfation, acetylation, and cross-linking with biodegradable polymers.103 These modifications create HA variants with controlled degradation rates, improved skin penetration, and targeted receptor interactions. One notable innovation is thiolated-HA, which forms disulfide bonds and exhibits superior muco-adhesion and antioxidative properties. Another advancement includes HA-polyethylene glycol conjugate copolymers, which extend the half-life and allow for sustained release in topical and injectable applications.104 These novel derivatives are being integrated into advanced formulations for wound healing, anti-aging, pigmentation control, and transdermal delivery systems, opening doors to multifunctional skincare and therapeutic platforms.105
Personalized cosmeceuticals
The concept of personalized cosmeceuticals is revolutionizing skincare by aligning HA-based products with individual skin biology, genetics, lifestyle, and environmental exposure. Advances in skin microbiome sequencing, gene expression profiling, and AI-driven diagnostics enable the creation of HA formulations that are customized for specific skin concerns such as dehydration, sensitivity, or premature aging.106 Companies are beginning to utilize smart diagnostic tools (mobile apps, skin scanners, and deoxyribonucleic acid kits) to assess individual skin profiles and deliver optimized HA-based products with precise MW, concentration, and added bioactive compounds. Personalized cosmeceuticals not only improve efficacy but also enhance consumer satisfaction and compliance. The integration of machine learning and big data analytics into dermatology is expected to significantly shape the next phase of HA-based skincare innovations.107,108
Role of hyaluronic acid in skin microbiome regulation
Emerging studies suggest that HA may play a pivotal role in regulating the skin microbiome, a critical factor in maintaining skin health and barrier function.109 HA can serve as a prebiotic, promoting the growth of beneficial skin flora while reducing pathogenic colonization. Its moisturizing effects help maintain an optimal skin pH and hydration, which are essential for a balanced microbiota. Some modified HAs have been shown to enhance the production of antimicrobial peptides and improve epithelial immunity, thereby indirectly supporting a healthy microbial environment (Figure 6).110,111 Current research is exploring the synergy between HA and probiotic or postbiotic ingredients, leading to the development of microbiome-friendly HA formulations aimed at conditions like atopic dermatitis, acne, and rosacea.112 This area holds significant promise for the formulation of next-generation dermo-cosmetics that target not just the skin but its living ecosystem.113
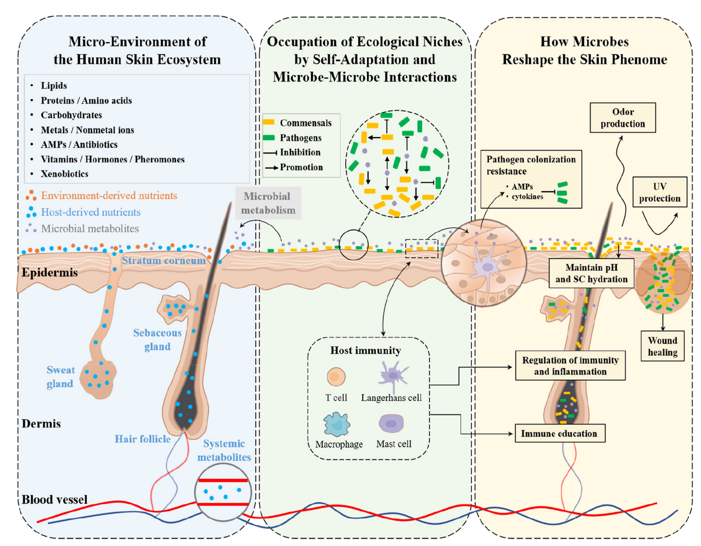
Figure 6 The skin functions as a dynamic ecosystem where the microbiome, metabolome, and phenome are closely interconnected. (Blue box) Diverse substances, derived from the host (stratum corneum, skin appendages, and plasma), environment (xenobiotics) and microbial metabolism, cover the skin surface, forming the micro-environment for skin microbiota;114 (Green box) occupation of ecological niches by self-adaptation and microbe-microbe interactions, promoting commensals or inhibiting pathogens; (Yellow box) the skin microbes, by their structures or bioactive molecules, reshape the host skin phenotypes.115
HA has emerged as a key component in modern cosmeceuticals due to its exceptional hydrating properties, biocompatibility, and multifunctional biological activity. This review compiles substantial evidence that highlights HA's essential role in maintaining skin hydration, promoting collagen synthesis, controlling inflammation, and supporting epidermal barrier repair. Its ability to interact with specific cellular receptors, such as CD44 and RHAMM, enables it to actively participate in skin regeneration and anti-aging processes. Recent advances in formulation science, including MW engineering, nanocarriers, cross-linking, and microneedle technologies, have substantially improved HA’s bioavailability and efficacy. Furthermore, HA’s synergy with other cosmeceutical agents like peptides, retinoids, and niacinamide enhances the scope of its therapeutic benefits, making it a central ingredient in both topical and injectable skincare products. Innovations such as next-generation HA derivatives and personalized cosmeceuticals driven by AI and genomic profiling signal a transformative advancement in HA-based skincare. Additionally, HA’s potential role in regulating the skin microbiome opens new frontiers in dermatological therapy. Given its robust safety profile, proven clinical benefits, and evolving technological capabilities, HA continues to redefine standards in cosmetic dermatology and therapeutic skincare. Future research should focus on personalized formulations, standardized labeling, and expanded clinical validation to further establish HA’s position in evidence-based cosmeceutical development.
None.
The authors declare that there is no conflict of interest.
None.

©2025 Gosiya,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.