Journal of
eISSN: 2574-9943


Research Article Volume 8 Issue 3
1Galderma Laboratories (Dallas, USA)
2Center for Tissue Engineering, Plastic Surgery Department, University of California, Irvine
Correspondence: Alan D Widgerow, Chief Scientific Officer Galderma, Head Skin Science Center for Innovation, Head Alastin Innovations, Division Chief, Research, Center for Tissue Engineering, Professor of Plastic Surgery, University of California, Irvine, Tel +1 949 394 5059
Received: September 02, 2024 | Published: September 16, 2024
Citation: Widgerow AD, Ziegler ME, Shafiq F, et al. Exosomes, peptides and peri-procedure management – comparative investigations. J Dermat Cosmetol. 2024;8(3):87-92. DOI: 10.15406/jdc.2024.08.00273
Introduction: Exosome technology is a promising new advancement in therapeutic options but is still in its early stages, leading to uncertainties. A small pilot study compared traditional peptide technology (Regenerating Skin Nectar with TriHex Technology® and TriHex Technology® peptide + Octapeptide) to two leading exosome products (Stem cell derived Lyophilized exosomes and Platelet derived exosomes) for post-procedure use. These studies were carried out on pre-clinical (ex vivo) and clinical front to investigate effects on the extracellular matrix, dermo-epidermal junction, and skin tolerance as post-procedure outcomes.
Methods and materials: The ex vivo model study was conducted by 3D Genomics using photodamaged skin from facelift patients. The skin was divided, processed under BSL2 conditions and cultured in transwells with specific media. Treatments, including peptides and platelet-derived products, were applied daily for 7 days, while stem cell-derived exosomes were applied as a single application as per the described usage. One set was left untreated as a control.
Skin samples were treated and analyzed using immunohistochemistry staining for tropoelastin and CD44. RNA was isolated after treatments and sequenced to assess changes in gene expression. The study aimed to compare the effects of different treatments on skin regeneration and tolerance. Clinically, the participants underwent microneedling of the face and applied exosomes and peptides on each side of the face as comparisons. Participants completed questionnaires on Day 0. On Days 0,1,2,3 and 4 participants completed their self-healing ratings and were also assessed by an assessor on their healing rating on a 5-point scale.
Results: The plated exosome products exhibited significant toxicity in the ex vivo model and neither of the exosome products produced relevant changes in gene expression or regenerative effects in this model. Participants in the small clinical series experienced unpleasant burning and stinging in keeping with the effects seen in the ex vivo model. In contrast, the peptide technology showed favorable gene expression upregulation and histological changes in the extracellular matrix and was well-tolerated and comfortable for post-procedure use, as has been demonstrated in multiple clinical trials.
Conclusion: The overall impression from these pilot studies suggests that peptide formulations are better tolerated and more effectively target ECM regenerative effects. Conversely, exosome preparations appear to be prone to skin reactions and lack clear targeting of skin regenerative pathways.
Keywords: exosome technology, skincare, post-procedure tolerability, facelift
Exosome technology provides an exciting new advancement in therapeutic options particularly related to specific disease processes. However, it is relatively early in its evolution compared to other treatment modalities, which creates uncertainties and challenges on various fronts. In skincare, in particular, the mechanisms of action and expected outcomes are ill-defined at present and validation of the technology have been almost exclusively limited to the presence of these vesicles and their size rather than the precise nature of their cargo and their expected function. In addition, the skincare indication has been extended to include peri-procedure management without good validation and practical confirmation of its efficacy and safety.
With that in mind, a small pilot study was carried out comparing traditional validated peptide technology (Regenerating Skin Nectar with TriHex Technology® (T1) and TriHex Technology® peptide + Octapeptide (TO) – Alastin® Skincare Carlsbad CA, a Galderma company) to that of two leading exosome products recommended for post-procedure use in the market. These exosome products were leading Stem Cell Derived Lyophilized Exosomes (Lyo) and leading Platelet Derived Exosomes (Pltd). Ex vivo effects related to extracellular matrix (ECM), dermo-epidermal junction and skin tolerance were investigated, and a small case series was also undertaken to observe and record post-procedure tolerability, comfort and outcome parameters.
Problems were identified with the exosome products relating to skin tolerance and a lack of discernable relevant changes in gene expression and regenerative effects in the ex vivo model. This was echoed in practical terms by unpleasant burning and stinging experienced by participants after the procedure. This is in strong contrast to the favorable ECM changes and gene expression elicited by the peptide technology in the ex vivo model and the tolerability, comfort and ease of use post-procedure with this technology as has been previously demonstrated in multiple clinical trials.1–4
Ex vivo model and investigations
All experiments were conducted by an independent laboratory at 3D Genomics (Carlsbad, CA) using an established ex vivo model.5 Photodamaged skin derived from patients undergoing facelift procedures was used (study approved under Veritas Institutional Review Board—study ID # 3192). Discarded facial skin received within 2 hours of surgery was used and processing was conducted under BSL2 laboratory conditions. The skin was washed in PBS, defatted, and hairs were shaved using a scalpel. The skin was then cut into approximately 5mm x 5mm square pieces and placed into transwells suspended in 6 well plates. About 2.0ml of Skin Media (DMEM/F12 Media, Adenine (50 uM), CaCl2 (1.88 mM), T3 Tri-iodothyronine (0.02 nM), Insu-lin-Transferrin-Selenium-Ethanolamine (ITS -X), Antibiotic-Antimycotic / Penicil-lin/Streptomycin 1%, 2% Heat Inactivated FBS, Glutagro 1%, and Gentamicin (0.01 mg/ml) was added to each well, and about 200ul to 300ul was added to each transwell to surround the skin sample while maintaining an air-exposed epidermal surface. Media was changed daily. The skin in the transwell cultures was kept under standard conditions in the 37˚ C 5% CO2 incubator for about 72 hours before initiating treatment.
All treatments were applied on the first day and samples were examined at 24 hours. Then the peptide and plated formulations were applied daily for 7 days. The stem cell exosome product was reconstituted as per company instructions and applied only once as directed. One set was left untreated as the baseline control. A total of 18 skin samples were processed, all from one individual. Twelve skin samples were used for the immunohistochemistry arm and 6 for the gene expression arm.
For the immunohistochemistry analysis, duplicate skin samples (from the same donor) were untreated or treated with T1, TO, Pltd, or Lyo. After 24 hours and at 7 days, the skin was fixed in formalin, paraffin-embedded, and processed to detect tropoelastin (red) (Elastin Products Co.) and CD44 (yellow) (Abcam). Goat anti-rabbit IgG Alexa647-conjugated, and goat anti-mouse IgG Cy3-conjugated secondary antibodies were used. The sections were counterstained with DAPI to detect the nuclei. Images were acquired using an immunofluorescence microscopy (Zeiss) to obtain a stitched image of the entire section.
For the gene expression arm, total RNA was isolated after the 24-hour and 7-day treatments. Bulk RNA sequencing was performed by Medgenome (Foster City, CA). The Takara SMARTer Stranded Total RNA-Seq Kit v3 - Pico Input Mammalian was used for the library preparation. Sequencing was performed on a NovaSeq sequencer with a 150 bp read length.
Clinical cases
Four participants opted to use Peptide and Exosome products on each side of the face post-microneedling procedures. Each participant received microneedling treatment of the face. Post- microneedling, first participant applied topical Stem Cell Derived Lyophilized Exosome product on one side of the face and applied TriHex peptide on the other side of the face. Second participant compared Pltd on one side with TriHex peptide on the other side of the face post-microneedling. Similarly, the two other participants were observed using Lyo exosome product on one side of the face and TO on the other side and Pltd on one side, comparing with TO on the other side, respectively. All participants completed questionnaires regarding their experience with the products and were also observed by an evaluator to evaluate their post-procedural rating of erythema, edema and crusting.
Participants completed questionnaires on day 0 using a 5-point Likert scale (1 = Strongly Disagree, 2= Disagree, 3= Neither agree nor Disagree, 4= Agree, 5= Strongly agree), right after application of products on each side of the face. Participants also completed self-assessment of healing at Days 0,1,2,3 and 4. An independent assessor also completed healing assessment rated each side of face at days 0,1,2,3 and 4 post-microneedling. Post procedure questionnaires at Day 0 (post-application of the products on each side of the face) included the following questions for each side of the face 1. The product did not sting upon application 2. The product did not burn upon application 3. The product soothes my skin 3. The product reduces skin discomfort (heat) 4. The product reduces pain 5. The product does not irritate my skin 6. The product makes my skin feel better.
At each visit (Days 0,1,2,3 and 4), Subject self-assessment of Healing assessments was completed for the redness, swelling, crusting, pain, heat and dryness on a 5-point scale (0= absent, 1= very mild, 2 = mild, 3 = moderate, 4= severe).
At each visit (Days 0,1,2,3 and 4), Assessments for Healing were completed by an assessor for erythema, edema, crusting and exudation on a 5-point scale (0=absent, 1= very mild, 2 = mild, 3 = moderate, 4= severe).
Ex vivo 24-hr. treatment
Before examining the results, it is important to note some initial observations related to the ex vivo model tolerance to these formulations. After 24 hours, the non-treated and both T1 and TO treatments appeared stable and in-line with our previous studies using this model. However, the ex vivo model responded poorly to the Pltd formulation after 24 hours with obvious toxicity manifesting as superficial sloughing of the sample. This was less obvious with the Lyo formulation. Moreover, the peptide technologies were stable through the 7-day treatment period. Twenty-four hours is a very early time point to observe changes in staining and responses at a biological level. However, the areas of anticipated response would be early migration of tropoelastin into superficial dermis and the dermo-epidermal junction (DEJ); stimulation of CD44 (biomarker for hyaluronic acid – HA- stimulation) and general observation of numbers and location of nucleated cells (DAPI stain). The untreated model (showing replicates) serves as a baseline. Untreated sample shows reasonable amounts of tropoelastin restricted to the dermis, minimal CD44 staining (mainly in the epidermis) and an unremarkable distribution of nucleated cells in the dermis and epidermis (Figure 1A).
The peptide treated samples both demonstrated good amounts of tropoelastin with migration into the DEJ in both treated groups (T1 and TO; Figures 1B and Figure 1C). For TO, there was a florid CD44 response, as indicated by the increased yellow signal in the dermis and epidermis representative of HA stimulation (Figure 1C). Healthy nucleated cells were observed in both treated samples and both demonstrated healthy ECM composition (Figures 1B - Figures 1C).
The samples treated with Lyo demonstrated tropoelastin mostly restricted to dermis without superior extension, no increase in CD44 staining or changes in nucleated cells (Figure 1D). These features were similar to untreated samples (Figure 1A). In contrast, the 24-hour images of the samples treated with Pltd show early signs of toxicity. Examining the dermis, a homogenous non-distinctive cellular appearance was obvious with the loss of architecture and while there was distinct yellow staining of the dermis, there was ill-defined cell connection by the CD44 staining. This was in contrast to other treatment groups as this non-specific distribution suggests free/unbound CD44 in a dysfunctional ECM (Figure 1E).
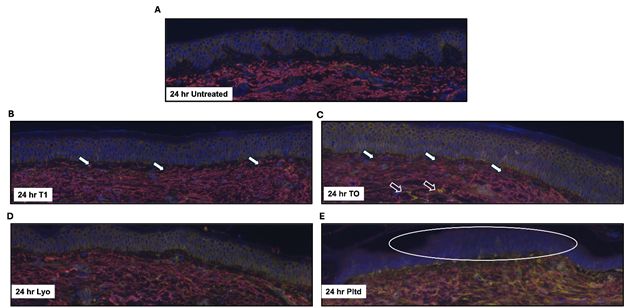
Figure 1 24-hour treatment assessment of elastin and CD44.
Discarded skin was cultured using the established ex vivo model and left untreated (A) or treated with T1 (B), TO (C), Lyo (D), or Pltd (E) for 24 hr. The tissue was processed, and sections were stained to assess tropoelastin (red) and CD44 (yellow) by immunofluorescence. DAPI was used to detect the nuclei (blue). Representative images are shown. The samples were prepared in duplicate, and representative images are shown. White-filled arrows indicate areas of tropoelastin migration in the DEJ. The open arrows indicate areas of HA stimulation. The oval represents poorly defined epidermal cells.
Ex vivo 7-day treatment: At day 7 after initiation of the study, the untreated sample showed a slight disruption at the DEJ, but the ECM and dermal elements were relatively intact and healthy (Figure 2A). Both peptide technologies (T1 and TO) at day 7 showed a good regenerative response. T1 (Figure 2B) showed relatively good architecture with a largely maintained DEJ and infiltration of tropoelastin into the superficial dermis and DEJ. This is particularly evident in the lower specimen (T1 (2)). For TO (Figure 6b), we observed a similar response for tropoelastin but again the CD44 staining was still dominant (Figure 2C).
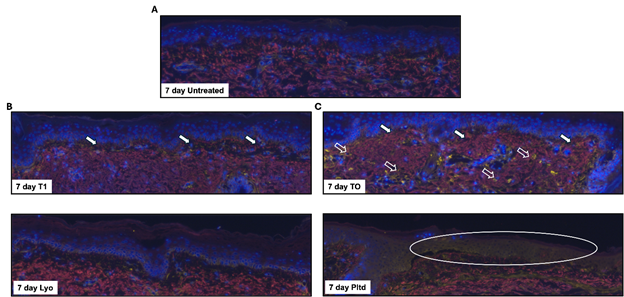
Figure 2 7-Day treatment assessment of elastin and CD44.
Discarded skin was cultured using the established ex vivo model and left untreated (A) or treated with T1 (B), TO (C), Lyo (D), or Pltd (E) for 7 days. The tissue was processed, and sections were stained by immunofluorescence to assess tropoelastin (red) and CD44 (yellow). DAPI was used to detect the nuclei (blue). Representative images are shown. The samples were prepared in duplicate, and representative images are shown. White-filled arrows indicate areas of tropoelastin migration in the DEJ. The open arrows indicate areas of HA stimulation. The oval represents an area of epidermal cell loss.
Although the Lyo-treated samples maintained a relatively good architecture [top sample Lyo (1) better than bottom Lyo (2)], there was relatively little new tropoelastin infiltration and limited CD44 staining (Figure 2D). Most of the Pltd sample has sloughed by day 7 with very few intact cells surviving at the surface and a disrupted architecture lower down (Figure 2E).
Gene expression changes (bulk RNA sequencing)
The correlation of gene expression profiles is used to aid in the biological interpretation of data. Since all the samples were from the same donor skin, they should show high correlation no matter the treatment. When comparing the different groups, it was apparent that at 24 hrs. and at day 7 the ‘Pltd’ group demonstrated the lowest correlation (differing color pattern) with any other groups, but the Pltd replicates were similar to each other (Figures 3A and Figures 3B).
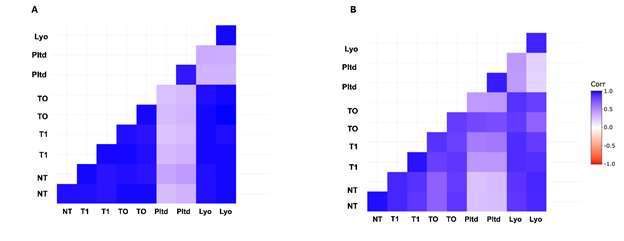
Figure 3 Protein coding gene expression correlation.
DESeq2-normalized counts of protein-coding genes were used to generate the correlations. Positive correlations are displayed in blue and negative correlations in red color. The colored legend shows the correlation coefficients and the corresponding colors. (A) 24 hr. treated samples (B) 7 day treated samples.
As far as gene expression is concerned, the significant differentially expressed genes with these formulations differed completely from group to group at 24 hrs. and at day 7. At 24 hours, the signature of the upregulated genes for T1 involved fibronectin matrix, cell cycle, notch signaling, endocytosis and keratinization, while the TO signature involved Wnt signaling, wound healing, PPARA expression, and lipophagy. The Lyo signature showed inhibition of phagocytosis and o-linked glycosylation of mucins, while the ‘Pltd’ group showed complement activation and cell death signaling (Figure 4).
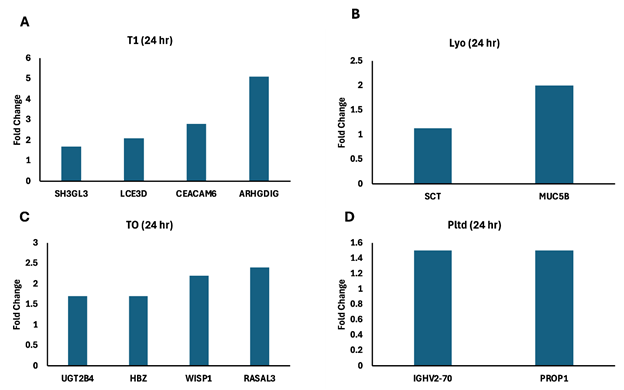
Figure 4 Significant differentially expressed genes 24 hr.
Discarded skin was cultured using the established ex vivo model and left untreated (A) or treated T1 (B), TO (C), Lyo (D), or Pltd (E) for 24 hr. RNA was extracted and RNA-seq was performed. The significant differentially expressed genes were subjected to the Reactome Database and the fold-change of those associated with a relevant pathway are presented.
Abbreviations: SH3GL3, SH3 domain containing GRB2 like 3, endophilin A3; LCE3D, late cornified envelope 3D; CEACAM6, CEA cell adhesion molecule 6; ARHGDIG, (Rho GDP dissociation inhibitor gamma; UGT2B4, UDP glucuronosyltransferase family 2 member B4; HBZ, hemoglobin subunit zeta; WISP1, cellular communication network factor 4 (CCN4); RASAL3, RAS protein activator like 3; SCT, Secretin; MUC5B, mucin 5B, oligomeric mucus/gel-forming; IGHV2-70, immunoglobulin heavy variable 2-70; PROP1, PROP paired-like homeobox 1.
At day 7, the gene expression profile for the T1 group demonstrated pathways involved in Wnt signaling, protein metabolism, and mTOR; while TO showed anchoring fibril formation, collagen crosslinking, cell-matrix interactions, and epidermis development. The Lyo gene expression profile pointed to keratinization and regulation of heat shock response, while Pltd showed Aquaporin-mediated transport and complement cascade (Figure 5).
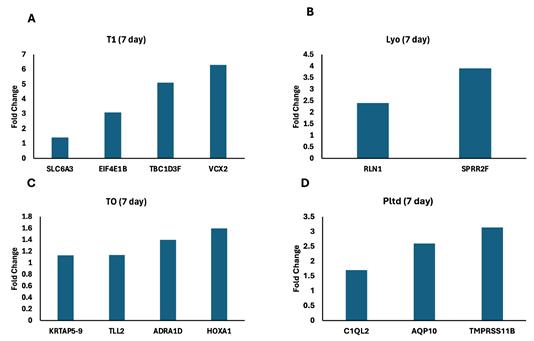
Figure 5 Significant differentially expressed genes 7 days.
Discarded skin was cultured using the established ex vivo model and left untreated (A) or treated T1 (B), TO (C), Lyo (D), or Pltd (E) for 24 hr. RNA was extracted and RNA-seq was performed. The significant differentially expressed genes were subjected to the Reactome Database and the fold-change of those associated with a relevant pathway are presented.
Abbreviations: SLC6A3, solute carrier family 6 member 3; EIF4E1B, eukaryotic translation initiation factor 4E family Member 1B; TBC1D3F, TBC1 domain family member 3F; VCX2, variable charge X-linked 2; KRTAP5-9, keratin associated protein 5-9; TLL2, tolloid like 2; ADRA1D, adrenoceptor alpha 1D; HOXA1, homeobox A1; RLN1, Relaxin 1; SPRR2F, small proline rich protein 2F; C1QL2, complement C1q like 2; AQP10, aquaporin 10; TMPRSS11B, transmembrane serine protease 11B.
Clinical case series results
Participant reported outcomes: At Day 0 post-microneedling, participant reported outcomes favored the peptide side across all questions after application of the products (Table 1).
|
Participant % agreement day 0 post microneedling product application |
||
|
Participant product questionnaires |
Peptide side |
Exosome side |
|
The product did not sting upon application |
100% |
0% |
|
The product did not burn upon application |
100% |
0% |
|
The product soothes my skin |
100% |
0% |
|
The product reduces skin discomfort (heat) |
75% |
0% |
|
The product reduces pain |
25% |
0% |
|
The product does not irritate my skin |
75% |
0% |
|
The product makes my skin feel better |
100% |
0% |
Table 1 Day 0 post-microneedling participant % agreement upon product application
100% of the subjects reported that the peptide side did not sting upon application whereas all reported stinging on the exosome side. 100% of the patients agreed that the product did not burn upon application on the peptide side and 0% on the exosome side (all experienced burning). 100% of the patients agreed that the product soothes the skin on the peptide side, and none reported that for exosome side. 75% of the patients reported that the product reduces skin discomfort (heat) on the peptide side, but none reported that for the exosome side. 25% reported the product reduces pain on peptide side and none reported that for exosome side. 75% participants reported that the product does not irritate their skin on the peptide side of the face, and none agreed for the exosome side. 100% of the participants agreed that the product made their skin feel better on the peptide side of the face and none agreed for the exosome side.
One participant reported that the Pltd product had a burning and stinging sensation upon application, whereas the side of the application of TO felt soothing. This was evident upon visual inspection Figure 6. This participant had to discontinue usage of the Pltd product on Day 2 due to unpleasant burning sensation.
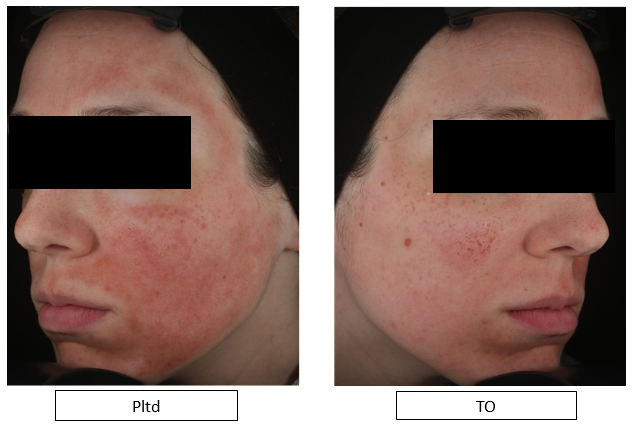
Figure 6 Day 1 post-microneedling, showing persistent erythema on the side of the Pltd application while erythema resolving on the side of the TO.
For subject self-assessment of healing, improvements across all parameters were reported on the peptide side as compared to the exosomes side.6
Self-assessment of healing
51% less redness with peptide on Day 1 compared to exosome side.
50% less on Day 2, and 67% less on Day 3.
86% less swelling on peptide side on Day 1.
Swelling completely subsided on peptide side by Day 2, while persisting on exosome side.
67% less pain on peptide side on Days 0 and 1.
Pain resolved on Day 2 on peptide side but persisted on exosome side.
40% less crusting on peptide side at Day 3.
67% less dryness on peptide side at Day 2.
40% less heat on peptide side compared to exosome side.
Assessor’s healing assessment
The healing assessments completed by the Assessor using the 5-point scale also reported favorable results on the peptide side overall.
4% less erythema on exosome side at Day 0.
42% less erythema on peptide side at Day 1.
31% less on Day 2, and 18% less on Day 3 on peptide side.
40% less edema on peptide side on Day 1.
55% less on Day 2, and 33% less on Day 3 on peptide side.
29% less crusting on peptide side on Day 2.
No exudation was observed for any participants.
Exosome technology constitutes a new advance in therapy for targeted disease processes. Of this, there is no doubt and as new actives within their cargos are identified and targeting becomes more precise, the technology will provide a new therapeutic alternative for many disease entities.7–10 The problem at this stage of development is that although claims are made related to efficacy and potency of formulations (particularly in skincare), these claims are based on the pure presence or absence of extravesicular bodies within a certain size range, with the assumption that the cargo that these vesicles carry is appropriate for the indication served. To date very few, if any of these companies have done comprehensive analyses of the actual cargo contents – the nucleic acid including miRNAs, lipid, proteins, and the multitude of variable content that can be carried by the exosomes. This may well relate to the fact that creating consistent batches of cargo within these exosomes may be extremely difficult and ensuring consistency in results from batch to batch may prove very challenging. In addition, keeping biologics active and stabilized, protecting against changes in pH, temperature and local environments, may mean the addition of preservatives and stabilizing agents that may not be ‘skin friendly’. Thus, a multiple of challenges still wait to be adequately answered including sourcing of the exosomes, their isolation, protection, cargo content, some unanswered safety concerns and a real defining of the outcome sought for different indications. All in all, this makes the current formulations used in peri-procedure skin care and maintenance untargeted, unvalidated and unpredictable.
With that as a background, we set out trying to understand some of the nuances involved in the use of these formulations on the skin and as adjuncts to procedures. We used a basis of comparison time and clinically tested peptide formulations that have been designed with specific targets in mind, created in nonaqueous form to be skin friendly, particularly related to vulnerable skin following procedures. Although limited information can be gleaned from aspects of this study related to nuances of these formulations, there are interesting new questions that arise, the primary one being, do we abandon time tested well researched synthetic actives in formulations for the shiny new biologic toys or do we advance the science further or look for alternatives that are safer, more targeted and predictable?
Analyzing this particular study, the relevant discussion points need to cover – formulation stabilization technologies and their immediate effect on skin; targeted approaches to skin maintenance and procedures, and the attainment of predictable results.
As far as formulation stabilization nuances are concerned, there is no doubt that something in the ‘Pltd’ formulation was not friendly to the ex vivo model and in fact to the client skin in the clinical series post-procedure. This did limit the conclusions that can be drawn from gene expression analyses as the upregulated genes were likely as a result of toxicity toward the model (complement cascade, cell death signaling). The loss of architecture in the 24 hour and particularly day 7 images bore out this theory. It does however raise the question of whether something in the stabilization process may not be skin friendly. Does this relate to a host of preservatives that are known to react with skin in other studies that we have undertaken with other formulations in the past?11 Although clinical studies have reported improved skin health in published reports, the analysis of the ‘best results’ or a ‘structured sub analysis utilizing the 75th percentile’ (that is 14 out of 56 test participants) which does throw into question the validity of the conclusions.12 The Lyo formulation appeared to be more ‘skin friendly’. Whether this relates to the fact that multiple peptides are in this preparation, or if stabilization routines are different, are open to speculation. However, this did allow us to dig a little deeper into skin targeting aspects.
One of the dominant themes in the TriHex technology® formulations has always been ECM modulation, clearance of old waste products (fragmented collagen, elastin, AGE products etc.) and replacement with new regenerative ECM components.2–4,13,14 The 2 variations tested in this trial involved the regular Tripeptide-1 and Hexapeptide-12 combination in T1 and the same combination with TO comprising the addition of a novel octapeptide-45, proprietary to Alastin® Skincare, discovered in-house.15 This new peptide was found to be very effective in stimulating intrinsic hyaluronic acid within the skinlayers.16 From a high level perspective, the peptide technologies show major advantages – skin friendly in ex vivo and clinical cases; predictable consistent batch formulations and outcomes, multiple prior validation studies documenting biologic and histologic changes and clinical outcomes; and very importantly, a targeted functionality involving ECM and regenerative changes documented in previous studies but confirmed once again in this study, with both variations demonstrating pathways intricately involved in ECM remodeling. This is in stark contrast to the Pltd formulation, which really did not allow a realistic assessment due to the effect on the tissue, and the Lyo formulation, which really showed no skin functionality targeting at all.
From a clinical perspective, this case series is too small to draw definitive conclusions, but it is enough to indicate client skin tolerance and overall experience to these topical formulations. This is an important observation as many of these superficial procedures are repeated and an unpleasant memory of burning and stinging does decrease the willingness of a client to redo a procedure or use the desired topical product post-procedure. In addition, it must be considered that some of these procedures have local anesthesia active for the first few hours and the real discomfort often only occurs on later exposure to the topical product, so assessment of skin tolerance needs to be immediately but also after a few applications of topical formulation.
Limitations of this study include an inability to truly assess the mechanism of action of the Pltd product due to tissue toxicity and reactions. This is an important element to uncover and appears to be mirrored in clinical experience. The numbers in the clinical cases are small but this was undertaken to see if the skin unfriendliness seen in the ex-vivo component also occurred in the clinical context. Interestingly this seemed to occur with both ‘biologic’ formulations begging the question of factors in formulation need to be improved before using these products as daily skin applications. Monitoring of adverse events will be critical.
New advances in science are exciting and should be embraced. At the same time, safety aspects, efficacy validation and predicted outcomes should be clearly defined. This study compared validated peptide technology with 2 exosome-based formulations using ex-vivo model histological assessment as well as gene expressions at 24 hours and 7 days, and a small case series comparing the products. The overall impression gained is that peptide technology is skin friendly with targeted ECM regenerative effects, while the exosome preparations exhibit some problematic skin reactions with no obvious targeting of skin regenerative pathways. More research needs to be done for these new technologies to compete with tried and tested formulations that are targeted, validated and predictable.
Drs Su Yang and Ted Choi from 3D Genomics for ex-vivo model set up and investigations.
Funding was provided by Alastin Skincare®, a Galderma company.
All authors are employees or consultants at Galderma.

©2024 Widgerow, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.