Journal of
eISSN: 2574-9943


Research Article Volume 2 Issue 6
1Laboratório de Pesquisa, Tecnologia em Cosméticos, FATEC Diadema-Luigi Papaiz, Brasil
2Laboratório de Pesquisa do Curso de Farmacia, Faculdade de Medicina do ABC, Brasil e LESIFAR Laboratório Escola Semi Industrial de Farmácia, Universidade Santo Amaro, Brasil
Correspondence: Carla Aparecida Pedriali Moraes, FATEC Diadema – Luigi Papaiz, Laboratório de Cosmeticos, 483 Luiz Merenda Avenue, Diadema, 09931-390 São Paulo – SP, Brazil, Tel +5501 14 09 22328
Received: August 16, 2018 | Published: November 20, 2018
Citation: Maia CS, Gama RM, Moraes CAP. Evaluation of efficacy of permanent hair dyes–intense red. J Dermat Cosmetol. 2018;2(6):113-116. DOI: 10.15406/jdc.2018.02.00099
Introduction: The aim of this work was to evaluate persistence of red-colored hair dyes and possible damage caused due to protein losses and due to hair thermal resistance.
Methodology: All analyses were carried out by means of diffuse reflectance spectrometry, thermogravimetry and derivative thermography (TG/DTG); color measures were assessed using the CIELCH (L*C*h*) scale.
Results and discussion: Results demonstrated that dye B on virgin and discolored hair caused greater protein losses (202.49% and 51.86%, respectively) than dye an on virgin and discolored hair (93.67% and 38.11%, respectively). Thermogravimetry analysis demonstrated that dye A had a higher thermal resistance to protein denaturation than dye B on both virgin (difference of 4.63ºC) and discolored (difference of 2.42ºC) hair. Results also demonstrated that dye a faded faster than dye B, as spectrophotometric analysis showed dye B persisted throughout 15 washes and also had lower values for luminosity and saturation than dye A.
Conclusion: As dye A had higher values of saturation and luminosity, color fading became perceptible after 10 washes.
Keywords: hair dye, color persistence, hair color fading, protein loss
Oxidative dyes are complex formulations, as they must provide intense and persistent color to hair; at the same time, their compounds must ensure hair fibers suffer the least amount of damage.
The human hair is constituted by four main structures: cuticle, cell membrane complex, cortex and medulla with its subunits.1,2 The cuticle is the most external structure, acting as a barrier protecting cortex and medulla from chemical substances.1‒3 The cell membrane complex is responsible for cell cohesion, while the cortex confers mechanical properties to the hair fiber.1,2
Permanent oxidative hair dyes are constituted of three main components: an oxidizing substance in an alkaline environment (hydrogen peroxide and ammonia), precursor compounds and coupling agents. Precursor compounds are oxidized by the oxidizing substance leading to release of oxygen, and then are combined with coupling agents producing the desired color. The molecular size of this complex must be high enough for it not to be easily released by the cortex.4 Permanent hair dye is the only type of dye capable of lightening and darkening the natural color of hair up to four tones; this happens due to opening of cuticles, which can also, on the other hand, lead to reduction of hair softness, resistance and brightness, causing the hair to look “unhealthy”.5,6
The aim of this work was to evaluate persistence of red hair dyes and possible damage caused to hair fibers due to protein loss and due to thermal resistance. Analyses were carried out using diffuse reflectance spectrophotometry, thermogravimetry and derivative thermogravimetry (TG/DTG); color measures were determined using the CIELCH (L*C*h*) scale. Permanent dyes which confer intense red color were preferred for studying, as their fading speed in comparison to other dyes are faster and there is still a lack of studies in scientific literature dedicated to investigation of such types of dyes.7
Materials
Due to commercial reasons, brand names were not divulged and thus were labeled simply A and B. The kits for dye A and B consisted of a flask containing oxidizing substance of 20volumes, a tube for cream color, a sachet containing a concentrate made of fruit and flower oils (dye A) and a sachet containing mask for conditioning treatments (dye A and B). All other reagents were of analytical grade and were purchased from either Sigma® or Merck®.
Sample preparation and discoloration
A total of 30hair samples obtained from Asian individuals were used in the study; they weighted about 1g each and had approximately 20cm of extension each. Half of the hair samples were subjected to a discoloration procedure. For such, a paste containing a mixture of 100mL hydrogen peroxide (volume 30) and discoloring powder (50g) at a 2:1 ratio was used.8
Dye application and washing
Dye application was carried out according to manufacturer instructions: the contents of the tubes containing hair dye were mixed with the contents of the flask containing oxidizing agent (20volumes); the sachet containing oils was mixed was well when the kit A was used. A total of 5.0g of the mixture was applied to hair samples, which were allowed to rest for 30minutes. Next, the hair samples were washed with water at 37.0±5.0°C for some seconds. A solution of 15% sodium lauryl sulfate (2mL) was then applied to hair samples by gentle massaging locks of hair with fingertips for 30seconds. Hair samples were again washed with water flowing at a rate of 1.8L/min until all detergent was removed. Hair conditioning substances present in the kits were used to condition locks of hair, which were then again washed and allowed to dry at room temperature under a relative humidity of 60%±5% for 48hours.1
Diffuse reflectance spectrophotometry and statistical analysis
Color of locks of hair was assessed with the aid of a reflectance colorimeter Konica Minolta CM 3700-A, model CIELCH (L*C*H*), capable of numerically representing the most recent color assessed, developed by X-Rite.
A total of five readings along the hair fiber were performed. Hair samples colored with hair dye A or B were washed 1, 5, 10 or 15times. Results were statistically analyzed with the aid of software OriginPro® 8.0 SR0 (OriginLab, Northampton, USA). Results obtained for luminosity (L*), red-green (a*) and yellow-green (b*) coordinates, chroma (c*) and hue (h*) were subjected to parametric analysis, the means corresponding to each parameter assessed being compared by univariate analysis (one-way ANOVA). Results for each sample were compared regarding variance between samples, standard-deviations and variations due to washes.9
Evaluation of protein loss
A total of 50mg of hair samples, weighted on an analytical balance (Marte–AD500–500g/1mg), were placed into a 250mL Erlenmeyer flask containing 15.0 mL of distilled water, which was then subjected to sonication using a TransSonic T460 (Elma – Brazil) for 40minutes. Next, the solution was filtered with filter-paper and a 2.0mL aliquot was used for quantification of protein loss. The Lowry method modified by Peterson used for quantification of proteins involves the transfer of 2.0mL of a stock-solution of albumin to test tubes, which is then mixed with solution a for 15minutes using a minishaker at 1000rpm, followed by a 10minute resting time. Next, 1.0mL of the previously prepared Folin-Ciocalteau reagent is added to the solution, which is then agitated for 15seconds followed by a 30minutes resting time, protected from light.10 The same procedures were carried out with previously processed hair samples. After reactions have ended, solutions were subjected to readings by UV-vis spectrophotometry (Biospectro SP) at 750nm.10,11 Readings for each dilution were carried out in quadruplicates; distilled water was used in order to obtain blank readings.
Thermogravimetry (TG) and derivative thermogravimetry (DTG) analysis
An amount between 0.5 and 1.0g of sample was placed in the thermal balance of the equipment (Shimadzu DTG-60); samples remained under dynamic air atmosphere (50mL.min-1) and were heated at a 10°C/min-1 ratio starting at a temperature of 25°C until an endpoint temperature of 800°C was reached. The methodology here employed is an adaptation of the method described by Gama (2010).10
Evaluation of color persistence
Figure 1 show results regarding analysis of color persistence in hue graphs. These images refer to wavelengths of both directly emitted and reflected light, as seen by the human eye: main colors red and blue and other colors as results of the mixture of this main colors.12
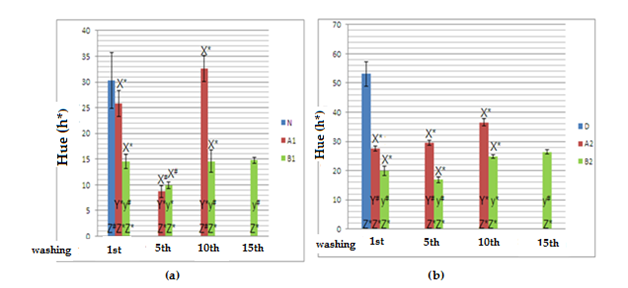
Figure 1 Hue graphs obtained from dyed virgin (a) and discolored (b) locks of hair.
N, natural hair; D, discolored hair; A1, virgin hair treated with dye A; A2, discolored hair treated with dye A; B1, virgin hair treated with dye B; B2, discolored hair treated with dye B; X, comparison between hair samples treated with dyes A and B; Y, comparison between hair samples treated with dye A throughout several washings; y, comparison between hair samples treated with dye B throughout several washings; Z, comparison between treated hair samples and the standard reference sample; # no statistical significance, considering α=5%; p ≤0,05, n=5 for all samples; *statistical significance, considering α=5%; p ≤0,05, n=5 for all samples.
Statistically significant differences were observed for samples A1 and B1 when assessing color fading at the 1st, 10th and 15th washings. These samples suffered darkening at the 5th washing. Hair samples colored with dye a suffered hair color tone variations as washings were carried out. No statistical differences were observed between samples at the 10th washing when compared to natural hair samples, indicating that the dye was being continuously removed throughout the washings. The colors of samples A2 and B2 (b) were statistically different; the color of samples A2 and B2 after the 10th washing when compared to the color of the same samples prior to previous washings was also statistically different (1st and 5th washings). The hue of sample A2 increased 22.0%, while this increase was of 25.73% for sample B2, but the average hue values for dye A were still 32.01% higher than the values for dye B. The resulting color when virgin hair was treated with dyes A and B was dark, as a result of the ability of the human eye to detect light and dark combined with color saturation. However, previously discolored locks of hair when treated with dye A were brighter. The final resulting red colors when locks of hair are treated with dyes A2 and B2 are very distinct from each other, the red color being more intense for dye A2 than dye B2 due to color saturation, as it influences the intensity by which the color is perceived by the human eye.
Evaluation of protein loss
Results obtained regarding protein loss demonstrated that dye A caused a lesser protein loss from the cuticle when applied to virgin (93.67%) or discolored (38.11%) hair than dye B when applied to virgin (202.49%) or discolored (51.86%) hair, when compared to standard samples N (100%) and D (100%). Samples D, A2 and B2 had a protein loss of 202.55%, 279.74% and 307.60%, respectively, when compared to a virgin hair sample (N). When compared to reference standards of discolored hair (D), the same samples had a smaller loss of protein, as described in Table 1; this happened due to previous discoloring damaging the hair fiber, which in turn allowed better penetration of the dyes as the structure of the cuticle had been weakened. Results regarding assessment of protein loss are described in Table 1.
Samples |
mg/g of hair |
% |
N |
0.03762 |
100 |
A1 |
0.07286 |
93.67 |
B1 |
0.1138 |
202.49 |
D |
0.0762 |
100 |
A2 |
0.10524 |
38.11 |
B2 |
0.11572 |
51.86 |
Table 1 Protein loss by hair samples as assessed by the Lowry method
Abbreviations: N, natural hair; D, discolored hair; A1, virgin hair treated with dye A; A2, discolored hair treated with dye A; B1, virgin hair treated with dye B; B2, discolored hair treated with dye B
Thermal analysis
Results regarding thermal analysis are described in Table 2.The first event refers to loss of water, which occurred at temperatures ranging from 55.47 to 65.45°C; loss of mass (loss of locks of hair) varied from 9.70 to 12.32%. The second event was observed at temperatures ranging from 232.07 to 236.52°C, and respective loss of mass varied from 19.06 to 23.67%, as demonstrated in Table 2.
Samples |
mg of hair |
1st Event Loss of mass (%) |
1st Event Temperature peak (°C) |
2nd Event Loss of mass (%) |
2nd Event Temperature peak (°C) |
N |
6,95 |
-9,70 |
65,45 |
-21,83 |
235,83 |
A1 |
8,86 |
-11,23 |
63,23 |
-22,47 |
235,75 |
B1 |
10,87 |
-9,57 |
66,37 |
-23,67 |
235,09 |
D |
8,15 |
-12,32 |
59,16 |
-19,06 |
236,52 |
A2 |
6,08 |
-9,87 |
56,70 |
-23,54 |
232,07 |
B2 |
5,67 |
-11,35 |
55,47 |
-22,57 |
234,10 |
Table 2 Results obtained from thermal analysis at 1st and 2nd events
Abbreviations: N, natural hair; D, discolored hair; A1, virgin hair treated with dye A; A2, discolored hair treated with dye A; B1, virgin hair treated with dye B; B2, discolored hair treated with dye B
Thermogravimetry results evidenced dye A had a higher resistance to temperature (A1 9.57% at 66.37°C and A2 12.32% at 59.16°C) than dye B (B1 9.87% at 56.70°C and B2 11.35% at 55.47°C). It was also verified that dye A1 was more resistant to temperature than virgin hair by itself (N) (9.78% at 65.45°C). The greatest difference of loss of mass in percentage between samples was of 22.32%, and the greatest temperature difference was of 10.9°C (55.47 to 66.37°C). The second event refers to keratin denaturation at the beginning of thermal degradation; at this point, the temperature reached was close to 235°C. Natural hair and discolored hair had losses of mass of 21.83% and 22.47%, respectively. A1 and A2 samples had losses of mass of 23.67% and 19.06%, respectively. Samples B1 and B2, on the other hand, reached lower temperatures, 232.07°C (23.54%) and 234.10°C (22.57%), respectively. The loss of mass was more evident at the second event due to keratin degradation, which is the most abundant protein found in hair fibers.13,14
The results here shown demonstrated that hair samples treated with dye A had their color fade sooner than samples treated with hair dye B. This might have happened because the kit for dye a contained 3.2mL of oils to be added during the mixing process at a proportion of 1:1 (50mL of hydrogen peroxide to 50g of dye). These oils are film making hydrophobic emollient substances which interfere with the diffusion of dying substances through hair fibers, compromising the coloring process.15
The kit for dye B, on the other hand, has a third more oxidizing agents than the kit for dye A, which probably caused formulation B to thoroughly react with melanin and keratin, allowing the colored polymer to be formed inside the cortex of hair fibers more efficiently. As shown in Figure 1, this caused virgin hair samples treated with dye B to remain colored even after the 15th wash.
Discolored locks of hair had higher color saturation, as these samples lacked natural color pigmentation. It was also possible to observe that even though the dyes used are standardized, as both are labelled 6.6 – intense red, the resulting color on hair samples was different possibly due to the different composition of precursor and coupling agents found in each formulation. Routine procedures, such as washing, can damage hair fibers, be them virgin or colored, due to lipid removal. Use of hair dryers and straighteners, due to functioning at excessive temperatures (nearly 200°C), compromises the integrity of proteins which compose the structure of hair fibers.5 Analysis of protein loss evidenced that the coloring process damages hair fibers; the greater the damage caused to the hair cuticle, the greater the loss of proteins.15 Dye B caused a greater protein loss than dye A due to the higher amount of hydrogen peroxide in its formulation.
The analyses of protein loss and thermal resistance were carried out in order to evaluate the actions of conditioning agents present in each hair dye kit assessed in this study. The use of conditioning agents in oxidative dyes aims to reduce the damage caused to hair fibers.14,15 The study performed by Gama (2010)10 demonstrated that active compounds such as silanetriol, pantenol, PEG-12 and dimeticone have thermo protective properties. Conditioning agents, such as cationic and ammonia quaternary tensioactives, cationic polymers and emollients found in both formulations assessed were probably the responsible for conferring resistance to temperature after the coloring processes had been carried out.
It can be concluded that hair colored with dye A suffered less losses of protein and had better thermal resistance due to the higher amount of conditioning agents found in the formulation, and also due to color fading faster. As for actual coloring, dye B was more efficient than dye A, as the color provided by it lasted longer. Even though it was tried to use hair dyes of same standardization (both are labelled 6.6 – intense red), the final color seen on locks of hair was different when comparing both dyes. Coloring applied to discolored hair allowed for better visualization of the red color when compared to coloring applied to natural hair.
The authors acknowledge the National Council for Scientific and Technological Development (CNPq) for providing financial aid (PIBITI – Initiation to Technological Development and Inovation Scholarship); Professor Henrique Eise Toma and Alcei Totti Silveira Junior (IQ-USP) for kindly providing the equipments used for thermogravimetry analysis; Professor Glauco Fioranelli Vieira and Eric Mayer Santos (FO-USP) for providing the diffuse reflectance spectrophotometer.
Authors declare that there is no conflict of interest.

©2018 Maia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.