Journal of
eISSN: 2574-9943


Research Article Volume 6 Issue 1
1Clínica Geisa Costa, São Paulo, Brazil
2Federal University of Maranhão, Brazil
3Clínica Lígia Colucci, Belo Horizonte, Brazil
Correspondence: Geisa Costa, MD, Avenida das Nações Unidas, 17.007, Torre Alpha – 10º andar – Conjuntos 101/102/103, Vázea de Baixo, São Paulo-SP, 04730-090, Brazil, Tel 55 11 5521.2446
Received: March 30, 2022 | Published: April 11, 2022
Citation: Costa GRM, Borges DC, Marques GG, et al. Dual-energy x-ray absorptiometry for evaluating body composition following cryolipolysis. J Dermat Cosmetol. 2022;6(1):23-27. DOI: 10.15406/jdc.2022.06.00202
Objectives: Cryolipolysis provides a nonsurgical treatment option for reducing excess subcutaneous fat. Although widely used, clinical outcomes such as photographs, patient questionnaires, and caliper measurements may be affected by subjectivity and operator-dependency. The current case series study evaluated changes in body composition using dual-energy X-ray absorptiometry (DEXA) in patients who underwent cryolipolysis treatment.
Methods: between 2019 and 2020, 5 patients underwent cryolipolysis at a dermatology practice in São Paulo, Brazil. Patients were treated in different body regions using a cryolipolysis medical device (CoolSculpting®; ZELTIQ Aesthetics, Inc., Pleasanton, CA, USA) with the CoolAdvantage™ or CoolAdvantage Plus™ vacuum applicators (ZELTIQ Aesthetics, Inc). Pre- and post-treatment, body weight and abdominal circumference were recorded. Fat and lean mass measurements were also obtained using a total body scanner.
Results: five patients (4 females, 1 male; mean age, 34 years) were treated in a total of 8 areas (2 cases in the flank, 3 cases in the abdomen, and 3 cases in the back). Three months post-treatment, body weight was reduced in 3 out of 5 (60.0%) patients, and abdominal circumference was reduced in 2 out of 3 (66.7%) patients. Most patients showed reductions in total fat mass (80.0%) and lean mass (60.0%). The percentage change in body weight was also correlated with the percentage change in total fat mass (R=0.81). Region-specific alterations in fat content were also observed.
Conclusions: most patients showed changes in body composition, including lower fat mass (5%-36% reductions) 3 months after cryolipolysis treatment. The results suggest that DEXA can be used to objectively visualize and quantify body composition changes following cryolipolysis treatment.
Keywords: body contouring, subcutaneous fat, cryolipolysis, dual-energy X-ray absorptiometry (DEXA)
The aesthetic desire to reduce excess fat has contributed to the popularity of body contouring procedures.1 Although liposuction is an effective treatment option, it remains an invasive procedure associated with surgical risks, such as complications from anesthesia and infection.2 Nonsurgical fat reduction provides a cost-effective option with faster recovery times than liposuction.2,3 According to the American Society for Dermatologic Surgery (ASDS) consumer survey and the American Plastic Surgery (APS) statistics, nonsurgical fat reduction is one of the most popular minimally invasive procedures performed in 2019.1,4 Some examples of nonsurgical modalities, include high-intensity focused ultrasound, unipolar radiofrequency, laser therapy, acoustic wave therapy, and cryolipolysis.3,5
Based on the concept that lipid-rich adipocytes are more susceptible to cold injury compared with surrounding tissues (e.g., skin), cryolipolysis uses intense, controlled, and slow cooling to induce subcutaneous fat cell apoptosis.3,5 Changes in subcutaneous fat are gradual and usually take 2 to 3 months for the fat volume in the treated area to be visible.3 The cryolipolysis medical device CoolSculpting® (Allergan, an AbbVie Company, Dublin, Ireland) was approved by the United States Food and Drug Administration (FDA) in 2010 for reduction of flank fat.3,6,7 Since then, the medical device has been shown to be safe and effective for the treatment of fat deposits in different body regions,8 including the submental and submandibular areas,9 upper arm,10 abdomen,11 back/bra fat,12 thigh,13 and buttocks (i.e., banana roll).12
In patients undergoing cryolipolysis, clinical outcomes are assessed both qualitatively, using photographic analysis and patient surveys/questionnaires, as well as quantitatively, using caliper and circumference measurements.8 There are limitations to these outcome measurements, including subjectivity and operator-dependency.8,14 Higher resolution ultrasound, magnetic resonance imaging (MRI), 3-dimensional (3D) imaging analysis, and diffuse optical spectroscopic imaging have been used to characterize changes in adipose tissue.8,15-19
Another imaging approach that can be used to evaluate body composition changes following nonsurgical fat reduction is dual-energy X-ray absorptiometry (DEXA). DEXA measures 3 principal components of the body based on their differential X-ray attenuation properties: bone mineral content, fat mass of soft tissues, and lean mass of soft tissues.20,21 This imaging technique is commonly used to diagnose and assess bone (e.g., osteoporosis), muscle (e.g., sarcopenia), and other metabolic disorders (e.g., obesity, lipodystrophy).21 Previous studies have shown the successful use of this technique to quantify adipose tissue changes in patients after surgical and nonsurgical fat reduction procedures.14,18,20,22 The current case series study evaluates changes in body composition (lean and fat mass) using DEXA in patients following cryolipolysis treatment.
Patients
Adult patients who presented with complaints of localized subcutaneous fat at a dermatology practice in São Paulo, Brazil, between 2019 and 2020 were included in the study. Patients were excluded from the study if they were pregnant or had autoimmune diseases, cold-related diseases, coagulation disorders, or umbilical/inguinal hernia. All patients provided informed consent prior to receiving any study-related treatment. The study conformed to the principles outlined in the Declaration of Helsinki.
Cryolipolysis treatment
After an initial consultation and physical examination, patients were treated in different body regions using a cryolipolysis device (CoolSculpting®; ZELTIQ Aesthetics, Inc., Pleasanton, CA, USA) with either the CoolAdvantage™ or CoolAdvantage Plus™ vacuum applicators (ZELTIQ Aesthetics, Inc.), with the CoolAdvantage Plus applicator being capable of removing a larger volume of abdominal and flank fat than the CoolAdvantage applicator.23 The procedure was performed for 35 minutes (CoolAdvantage applicator) and 45 minutes (CoolAdvantage Plus applicator) at a temperature of -11°C (for both applicators). After the procedure, the applicator was removed, and a manual 2-minute massage was performed according to the established treatment protocol. All procedures were conducted by a single experienced physician.
Outcome measurements
Prior to treatment and at a scheduled 3-month follow-up visit, body weight and abdominal circumference measurements (collected using a tape measure), as well as DEXA scanning to assess body fat composition were conducted. Clinical photographs were obtained under controlled conditions. Body composition measurements (e.g., total and regional fat and lean mass) were performed with a total body scanner (Lunar iDXA, GE Healthcare, Chicago, IL, USA). Changes in body weight, body mass index (BMI), abdominal circumference, and body composition measurements are presented as descriptive statistics. Cross-correlation analysis was performed using a Pearson’s correlation test.
Patient disposition, demographics, and baseline characteristics
Between 2019 and 2020, 5 patients (4 female, 1 male) with a mean age of 34 years (range, 26-42 years) completed a single treatment session. A total of 8 areas were treated in the 5 patients, including 2 cases in the flank, 3 cases in the abdomen, and 3 cases in the back Table 1.
Patient |
Age |
Sex |
Applicator used |
Treatment areas |
1 |
41 |
Female |
CoolAdvantage |
Flank (6), back (2) |
2 |
42 |
Male |
CoolAdvantage Plus |
Abdomen (4), back (2) |
3 |
34 |
Female |
CoolAdvantage |
Abdomen (2) |
4 |
26 |
Female |
CoolAdvantage |
Flank (6), back (2) |
5 |
27 |
Female |
CoolAdvantage Plus |
Abdomen (6) |
Table 1 Patient Demographics and Treatment Areas
Outcome measurements
Table 2 lists patient weight, BMI, and abdominal circumference measurements pre-treatment and during the 3-month follow-up visit. Three out of 5 (60.0%) patients lost body weight. Among these responders, the mean difference [diff] was -18.03 kg (range of -4.4 kg to -26.7 kg). Two out of 3 (66.7%) assessable patients showed lower circumference measurements (mean diff among responders of -4.5 cm and range of -3 cm to -6 cm). Representative pre-treatment and post-treatment photographs for each patient demonstrate reductions in fat after cryolipolysis Figures 1 & 2. All patients reported feeling satisfied with treatment outcomes.
Patient |
Body weight (kg) |
BMI (kg/m2) |
Circumference (cm) |
||||||
Pre-treatment |
Post-treatment |
Diff† |
Pre-treatment |
Post-treatment |
Diff† |
Pre-treatment |
Post-treatment |
Diff† |
|
1 |
97 |
74 |
-23 |
36.6 |
27.5 |
-9.1 |
88 |
85 |
-3 |
2 |
123.7 |
97 |
-26.7 |
37.3 |
29.6 |
-7.7 |
NA |
NA |
|
3 |
71.5 |
67.1 |
-4.4 |
23.9 |
22.4 |
-1.5 |
NA |
NA |
|
4 |
91.3 |
98 |
6.7 |
31.9 |
34.3 |
2.4 |
104 |
98 |
-6 |
5 |
84.4 |
85 |
0.6 |
33.8 |
33.2 |
-0.6 |
95 |
101.1 |
6 |
Table 2 Changes in Body Weight and Abdominal Circumference
NA indicates that patient data were not available.
†Diff is calculated by post-treatment minus pre-treatment.
BMI, body mass index; Diff, difference.

Figure 1 A 34-year-old female underwent cryolipolysis in her abdomen (2 cooling treatments). (A) Representative patient photographs before and approximately 3 months after treatment. (B) Dual-energy X-ray absorptiometry (DEXA) scan images before and approximately 3 months after the procedure. Fat mass (colored image) scans are shown.
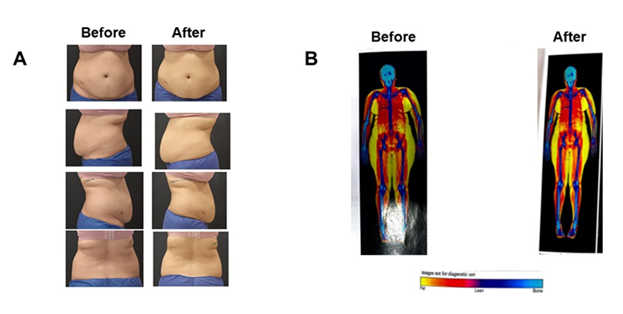
Figure 2 A 27-year-old female underwent cryolipolysis in her abdomen (6 cooling treatments). (A) Representative patient photographs before and approximately 3 months after treatment. (B) Dual-energy X-ray absorptiometry (DEXA) scan images before and approximately 3 months after the procedure. Fat mass (colored image) scans are shown.
When assessed by DEXA during the post-treatment visit, 4 out of 5 patients (80.0%) demonstrated lower total fat mass (mean % diff among responders of -20.88% and range of -5.06% to -35.50%), and 3 out of 5 (60.0%) patients showed lower total lean mass (mean percentage diff among responders of -10.33% and range of -4.96% to -16.85%) Table 3. When subdivided by region, fat mass was reduced in the arms of all 4 assessable patients (100%) and in the legs of 4 out of 5 (80.0%) patients, but not in the trunk area Table 4.
Patient |
Fat mass (g) |
Lean mass (g) |
||||||
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
|
1 |
45642.8 |
29441 |
-16201.8 |
-35.5 |
46189.2 |
41944 |
-4245.2 |
-9.19 |
2 |
46252.7 |
34129 |
-12123.7 |
-26.21 |
71299.1 |
59287 |
-12012.1 |
-16.85 |
3 |
27311 |
22737 |
-4574 |
-16.75 |
41647 |
41949 |
302 |
0.73 |
4 |
44431 |
42181 |
-2250 |
-5.06 |
43332 |
43424 |
92 |
0.21 |
5 |
45697 |
54231 |
8534 |
18.68 |
33443 |
31783 |
-1660 |
-4.96 |
Table 3 Changes in Body Composition
†Diff is calculated by post-treatment minus pre-treatment.
‡% Diff is calculated using the following: (Diff / pre-treatment)
*100.
Diff, difference.
Fat mass (g) |
Lean mass (g) |
|||||||
Arms |
||||||||
Patient |
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
1 |
4109.1 |
2607 |
-1502.1 |
-36.56 |
4091.8 |
4185 |
93.2 |
2.28 |
2 |
4301.0 |
3080 |
-1221.0 |
-28.39 |
8015.7 |
6989 |
-1026.7 |
-12.81 |
3 |
1956 |
1705.0 |
-251.0 |
-12.83 |
3271 |
3480.0 |
209.0 |
6.39 |
4 |
6966 |
4734 |
-2232 |
-32.04 |
5802 |
4630 |
-1172 |
-20.2 |
5 |
NA |
NA |
3629.0 |
3287 |
-342.0 |
-9.42 |
||
Legs |
||||||||
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
|
1 |
16516.9 |
12091 |
-4425.9 |
-26.8 |
14805.2 |
13714 |
-1091.2 |
-7.37 |
2 |
15368.9 |
10169 |
-5199.9 |
-33.83 |
22356.0 |
20384 |
-1972.0 |
-8.82 |
3 |
13515 |
10989.0 |
-2526.0 |
-18.69 |
15096 |
14848.0 |
-248.0 |
-1.64 |
4 |
17120 |
16448 |
-672 |
-3.93 |
15860 |
15584 |
-276 |
-1.74 |
5 |
18706 |
20957 |
2251.0 |
12.03 |
11363.0 |
12455 |
1092.0 |
9.61 |
Trunk |
||||||||
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
Pre-treatment |
Post-treatment |
Diff† |
% Diff‡ |
|
1 |
NA |
NA |
NA |
NA |
||||
2 |
25132.9 |
NA |
36962.5 |
NA |
||||
3 |
5450 |
9207.0 |
3757.0 |
68.94 |
10276 |
20499.0 |
10223.0 |
99.48 |
4 |
19227 |
20009 |
782 |
4.07 |
18390 |
20004 |
1614 |
8.78 |
5 |
22475 |
26209 |
3734 |
16.61 |
15476 |
12067 |
-3409 |
-22.03 |
Table 4 Region-specific Changes in Fat Mass as Measured Pre- and Post-Treatment
NA indicates that patient data were not available.
†Diff is calculated by post-treatment minus pre-treatment.
‡% Diff is calculated using the following: (Diff / pre-treatment)
*100.
Diff, difference.
Relationship between outcome measurements
Of the 3 assessable patients with abdominal circumference measurements, patient 1 showed both body weight loss and a reduction in abdominal circumference 3 months post-treatment, while patient 5 did not show body weight loss and a reduction in abdominal circumference. In contrast, patient 4 did not lose weight despite a reduction in abdominal circumference. Important to note that weight loss may have contributed to the results. Of the 3 patients that demonstrated weight loss (patients 1, 2, and 3), all showed lower total fat mass 3 months post-treatment. Of the 2 patients that did not lose weight, patient 4 showed lower fat mass while patient 5 showed higher fat mass. There was a positive correlation between the percentage change in body weight and percentage change in total fat mass (R=0.81) although this did not reach statistical significance.
Safety
Adverse events, including edema, bruising, and hematomas, were mild and resolved prior to the follow-up visit.
The current study underscores the ability of DEXA scanning to show qualitative and quantitative body composition changes in patients undergoing cryolipolysis. At the 3-month follow-up, 4 of 5 patients demonstrated reductions in fat mass, which ranged from 5% to 36%. The reductions in fat mass were within the range observed in previous studies, which used other outcome measurements.15,24,25 For the majority of the patients (4 out of 5), changes in fat mass followed the same patterns seen with body weight changes (3 patients showed reductions; 1 patient showed an increase). A positive correlation was observed between the percentage change in body weight and percentage change in fat mass, suggesting that lower fat mass, as a result of localized fat reduction using cryolipolysis, may contribute to overall changes in body weight. However, the sample size was small and changes in body weight were not controlled in the current study.
Two of the 5 patients showed weight gain during the 3-month follow-up visit. Fat is an endocrine organ, and the removal of fat may disrupt metabolic homeostasis, which may lead to weight regain.26 A prospective, single-arm study demonstrated that cryolipolysis reduced skinfold thickness in treated areas as measured by calipers, an indirect measure of subcutaneous fat, and improved patient satisfaction despite a lack of mean weight loss.23 Fluctuations in body weight despite reductions in fat after cryolipolysis treatment have also been observed in previous studies.6,27 Therefore, it is important to counsel patients that cryolipolysis is not a treatment for obesity, but, rather, one for the reduction of localized fat. Because lifestyle factors and nutritional habits may also contribute to weight gain,27 patients undergoing fat-reduction procedures should also be educated that weight loss/maintenance is accomplished through a nutritional diet and exercise.26 More in-depth studies investigating the effects of localized fat removal on metabolic homeostasis are needed.
How changes in body composition as measured by DEXA relate to other conventional outcome measurements (e.g., body weight, abdominal circumference, patient satisfaction, photographic analysis) may also need to be investigated in the future. Another potential question to investigate is how fat reduction in specific body regions (e.g., flank, abdomen, and back) contributes to region-specific body composition changes. A previous study observed systemic reductions in fat, even in nonexposed sites, after cryotherapy treatment.22
Although this study evaluates the use of DEXA analysis after cryolipolysis treatment, previous studies have reported its utility in assessing fat changes after surgical and nonsurgical fat reduction procedures.14,18,20,22 One advantage of DEXA scanning is the ability to quantitatively assess region-specific changes in fat content and distribution. The images obtained by DEXA allow objective and visible evidence of improvement after cryolipolysis.20 In the clinical setting, both the visualization and quantification of body fat using DEXA can provide patients with a clear idea of treatment progress, which may serve as continued motivation for them to maintain the results of treatment.
Some limitations include the small sample of patients evaluated, the absence of a control group and the lack of a maintenance of body weigh within a predetermine weight range. And incomplete data for some outcome measures, such as abdominal circumference. Unlike previous studies,6,27 the current study did not control for fluctuations in body weight; therefore, the observed changes may also result from other factors, such as lifestyle and diet, in addition to cryolipolysis. Although future patients may also be concerned about radiation exposure during DEXA scanning, which may subsequently limit the number of clinical endpoints or follow-up visits, the radiation dose of patients undergoing this procedure is generally low and similar to natural background radiation.14,20,21 Furthermore, compared with other imaging techniques such as computed tomography or MRI, DEXA is relatively cost effective.21
This research was funded by an unrestricted educational grant provided by Allergan Aesthetics, an AbbVie Company.
The authors have stated explicitly that there are no conflicts of interest in connection with this article.

©2022 Costa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.