Journal of
eISSN: 2574-9943


Research Article Volume 6 Issue 3
Discipline of Dermatology, Pontifícia Universidade Católica, Brazil
Correspondence: Nascimento C, Discipline of Dermatology, Pontifícia Universidade Católica, Brazil, Tel (62) 8199-0929
Received: June 16, 2022 | Published: August 22, 2022
Citation: Cunha MG, Bernardo ACS, Romani PI, et al. Acceptability and tolerability of new brand injectable product based on hyaluronic acid for lip rejuvenation Rennova Lips®. J Dermat Cosmetol. 2022;6(3):85-88. DOI: 10.15406/jdc.2022.06.00215
Currently, with the greater importance given to self-image, particularly with the phenomenon of “selfies,” the facial aesthetics segment has become more widely exposed, and with it, the shape, size and contour of the lips has gained greater prominence, leading more and more patients to seek to improve or correct features to reach the aspect considered ideal for the lip area, both in the media and among the general public. Many alloplastic products, both topical and injectable, are touted by the pharmaceutical industry for this purpose. The application of hyaluronic acid-based injectable products have the fewest adverse effects, being highly studied and with well-defined techniques for use. In this study, the hyaluronic acid product Rennova Lips®, recommended for use on the lips, was used, followed by an observation of its tolerability and acceptability. The satisfactory maintenance of results after 12 months of the procedure was noted, as well as low rates of adverse effects, qualifying the product for this indication.
Protocol approved by Ethic committee of the Pontifícia Universidade Católica- PUC- Sorocaba - Approval number: 46120821.6.00005373
This project was executed with self financial and donation of the products by Innova Pharma
The great demand for aesthetic procedures, mainly facial ones, has provided an increase in the knowledge of the topographical and dynamic anatomy of this area. The understanding of the aging processes and their modifications imposed on the regions of the face has led to the improvement of the techniques used for rejuvenation. Among the areas of attention, the lip region is of great importance, both in terms of contour and volume, as it denotes the appearance of youthfulness.1–3 The use of injections of products based on hyaluronic acid for volume correction and lip contour improvement, as well as for the treatment of perioral wrinkles, began in the mid-nineties and was the one that presented the greatest development, becoming increasingly secure and individualized. In addition to the knowledge of the anatomy of the labial region, the use of increasingly refined and specific products provides natural results with the greatest possible safety Figure 1.4–8
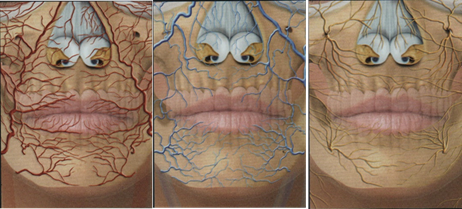
Figure 1 Schematic view of the arteriovenous and innervation network of the perioral region. In Braz9.
Objectives
To tolerability and acceptability of a new hyaluronic acid-based product specifically for use in the lip region (Rennova Lips®).
The universe of this study included eight female patients, aged between 39 and 75 years (mean age of 55 years), without comorbidities, who complained of dissatisfaction regarding lip volume and contour. After evaluation by the professional and verification of compliance with the established inclusion and exclusion criteria, the study was explained step by step, as well as its objectives. All patients were photographed before the procedure in the front, 45-degree and 90-degree positions, on both sides. Prior to the procedure, asepsis and antisepsis of the entire face were performed with 2% chlorhexidine in aqueous solution. Bilateral anesthesia of the infraorbital innervation was performed with infiltration of 0.1 ml of 2% lidocaine without a vasoconstrictor.
Subsequently, 0.5 ml of Rennova Lips® hyaluronic acid was applied to the upper and lower lips, with the aid of a 5 mm 27G microcannula, for volumetric correction of the lip region. At the end of the procedure, cold compresses of 0.09% saline solution were applied for 30 minutes.
Control visits were conducted on days 15, 30, 90, 180, 240 and 360 after the procedure. To assess the perception of product safety, the patients answered a questionnaire with questions that took into account variables such as the pain experienced by the patient, sensitivity in the treated area, tolerability to the treatment, texture of the lips, and appearance of nodules or lumps, in addition to satisfaction with the results and probability of repeating the procedure.
Lip filling is one of the most sought-after procedures by patients in a wide age group.10 Additionally, lip projection is often requested to resolve asymmetries or improve the harmonization of the face.11
In this study, eight female patients with complaints regarding lip volume and/or contour were treated with the product Rennova Lips®, distributed by Innovapharma and duly approved by the Brazilian regulatory agencies. As recommended by the literature, microcannulas were used for the procedure, thereby minimizing the occurrence of significant hematomas in the region.12,13
Figures 2 (a, b & c) & Figure 3 (a, b & c) show the esthetic improvement of the perilabial region, with the erasure of the thin “barcode” rhytids on the upper lip and the reduction of the labiomental fold near the corner of the mouth. The result observed at 6 months is adequate, with no stigmata related to overcorrection or anatomical dystocia. When comparing the 12-month result, it is noted that there was a slight partial loss of lip volume, but still maintaining satisfactory and natural results, without compromising the satisfaction of patients and observers.
To assess the safety of the product, variables such as the pain experienced by the patient, sensitivity of the treated area, tolerability to the treatment, texture of the lips and the presence of lumps or nodules were observed. Graph 1–4 show the comparison of each variable between the day of treatment and throughout the first week.
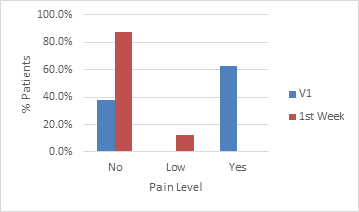
Graph 1 Comparison of the pain level reported by patients on the day of the procedure and throughout the first week.
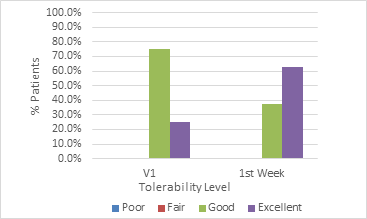
Graph 3 Distribution of the evolution of tolerability to the procedure in the lip area reported by patients.
Graph 4 Distribution of the evolution of tolerability to the procedure in the lip area reported by patients.
Pain during the procedure was reported by about 60% of patients. It is an expected complaint, considering that the lips are one of the most sensitive regions of the face, due to the richness of its nervous system, both sensory and motor, for performing mimicry, phonation and mastication activities.14–17 This percentage drops to less than 20% during the period evaluated, with approximately 10% of patients reporting minimal pain.
As for sensitivity, this variable was divided into levels ranging from 1 to 5, 1 corresponding to the absence of complaints and sensitivity and 5 corresponding to very high sensitivity. The patients’ responses can be seen in Graph 2.
The procedure tolerability grading was also evaluated to corroborate the product’s safety profile, as it provides an idea of the probability of adherence of patients regarding possible treatments of more than one session to treat more critical conditions, such as atrophies or deformities in the lip area. Graph 3 shows that the tolerability of the procedure is over 70%, reaching 100% among patients by the fifth visit. In addition to tolerability, patients were asked about the appearance of any other type of adverse effect such as lasting hematomas, nodules, lumps, asymmetries, etc.18 None of the patients reported any of these events.
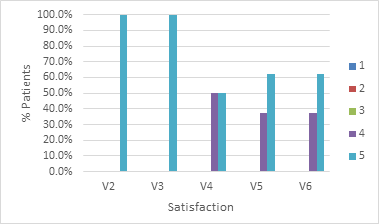
Study participants were also asked to rate their satisfaction regarding the results obtained and the likelihood and/or desire to perform the procedure again. These two questions were answered in each of the visits conducted, with the aim of observing the evolution over time of the perception regarding the product’s effectiveness. Graph 4 & 5 show the distribution of satisfaction and the desire to redo the procedure, respectively.
It is possible to observe that, in the first two post-procedure visits, 100% of the patients reported having maximum satisfaction with the results obtained. This percentage drops to half of the patients in the last two visits. It is believed that the cause is the absorption of the product with the partial loss of the volume obtained. Nevertheless, the remaining half reported a high degree of satisfaction (level 4). This result is also expected when considering the product’s resorption time, outcome expectations, and body perception of self-image. The answers obtained regarding the desire to redo the procedure are expected and consistent with the patients’ perception of the effectiveness of the product and can be seen in Graph 5.
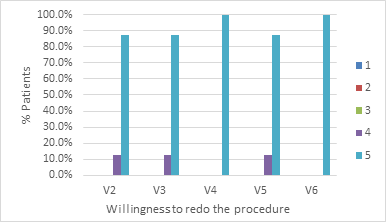
Graph 5 Distribution of the evolution of tolerability to the procedure in the lip area reported by patients.
As for the homogeneity and texture, all patients rated them as “excellent,” which demonstrates the safety of the procedure and the product. It is also important to emphasize that the observation of the correct techniques for performing the injection and knowledge of the anatomy of the area are paramount for the success of the procedure.19–22
The data in the graphs show that the patients were satisfied with the result. It can be seen that, after 90 days following the procedure, 87.5% of them state that they would redo the procedure immediately and 12.5% in the subsequent six months. The degree of satisfaction is also quite high in the indexed literature.23,24
The non-observation of serious adverse effects by patients and observers demonstrates the safety of Rennova Lips® for performing the lip-filling procedure.
None.
The author declares there is no conflict of interest.

©2022 Cunha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.