Journal of
eISSN: 2574-9943


Research Article Volume 4 Issue 5
Correspondence: Eric Andres, Oroxcell, 102 avenue Gaston Roussel, 93230 Romainville, France
Received: October 16, 2020 | Published: October 27, 2020
Citation: Andres E, Barry M, Hundt A, et al. A new prediction model for distinguishing skin sensitisers based on IL-18 release from reconstructed epidermis: enhancing the assessment of a key event in the skin sensitisation adverse outcome pathway. J Dermat Cosmetol . 2020;4(5):123-137. DOI: 10.15406/jdc.2020.04.00164
Objective: The aim of this study was the evaluation of the prediction capacities of the rhe/IL-18 assay for the identification of contact sensitisers using the EpiCSTM reconstructed epidermis model by applying a newly developed prediction model.
Methods: 20 references substances were tested following the rhe/IL-18 protocol. Each batch of reconstructed epidermis was characterised with respect to its basal level of IL-18 release by the inclusion of unexposed and solvent only controls. The data were interpreted using a new prediction model based on the ratio of the IL-18 concentration at maximum response to test item concentration. The prediction performance was compared with that of previously published prediction models based on the differential release of IL-18 compared to the solvent control.
Results: The EpiCS test system provided remarkably low and stable levels of basal and solvent induced IL-18 release over 10 batches of epidermises. The performance of the newly developed prediction model was found to be comparable to previously published results and differences were examined in terms of balance between positive and negative substance identification between the tried prediction models.
Conclusion: The results indicate that the prediction model provides promising performance when applied to the data of the rhe/IL-18 assay, with levels of sensitivity, specificity and accuracy similar to OECD validated assays, albeit obtained with a reduced number of substances. Therefore, further testing of a wider range of substances type should be envisaged.
Keywords: animal testing alternatives, toxicity tests methods, allergens toxicity, epidermis, drug effects, skin, keratinocytes, skin tests, sensitisation, interleukin-18 immunology, haptens, toxicity, epidermis, immunology, humans
AOO, acetone olive oil; AOP, adverse outcome pathway; BSA, bovine serum albumin; DPRA, direct peptide reactivity assay; EC50, half maximal effective concentration; EE, Epidermal equivalent; ELISA, enzyme-linked immunosorbent assay; EpiCSTM, reconstructed human epidermis from cellsystems laboratories; EpiDERMTM, reconstructed human epidermis from Mattek laboratories; GPMT, guinea pig maximisation test; h-CLAT, human cell line activation test; HRP: horseradish peroxydase; IL-18, interleukin 18; LLNA, local lymph node assay; LLOQ, lower limit of quantification; MTT, methyl thiazolyldiphenyl-tetrazolium bromide; OD, optical density; OECD, organisation for economic co-operation and development; PBS, phosphate buffer saline; PM, prediction model; rhe, reconstructed human epidermis; RHETM, reconstructed human epidermis from Episkin laboratories; TMB, Tetramethylbenzidine ; VUMC-EE, reconstructed human epidermis from VRIJE university medical center
Sensitisation evaluation has become an essential part of the safety assessment of substances in industry, especially the cosmetics industry.1–4 In the past, sensitisation was evaluated on rodents using the Local Lymph Node Assay5 or the Guinea Pig Maximisation Test.6 However, since March 2009, all in vivo assays have been banned in Europe for the evaluation of cosmetics raw materials, with the exception of specific endpoints such as repeated-dose toxicity, reproductive toxicity and toxicokinetics for which the date was extended to March 2013.7
In response, a plethora of in vitro assays were developed as alternatives to permit the toxicological evaluation of substances without the use of animals.8,9 Among these, specific tests were set up for the detection of sensitisation, with the main objective of distinguishing sensitising substances from non-sensitising substances.
Considerable research into the very complex mechanism of skin sensitisation has culminated in the characterisation of the sensitisation process with an adverse outcome pathway,10 consisting of four consecutive key events (KE). The identification of the 4 key events has permitted the refinement and targeting of in vitro assay development and testing toward the key events in the AOP.
The first key event of the skin sensitisation AOP is the penetration of the sensitiser through the stratum corneum, allowing the substance to react with skin proteins to become a hapten-protein conjugate which can potentially induce the biological part of the sensitisation process. The Direct Peptide Reactivity Assay (DPRA, OECDf 2015)11 specifically aims at assessing the haptenization potential of substances by measuring their reactivity with model peptides.
The second key event is the reaction of the keratinocytes to the hapten-protein conjugate.12 The keratinocytes react by releasing pro-inflammatory cytokines such as IL-18, which is specific to the skin sensitising process.9,13-15 Alongside the keratinocytes activation, the dendritic cells present in the epidermis are exposed to both the hapten-protein conjugate and to specific cytokines released by the keratinocytes. This co-exposure activates the dendritic cells, inducing a change of phenotype that enables their migration to the local lymph node. This constituting the third key event.8 Once localized in the local lymph node, the mature dendritic cells present the antigen to the T lymphocytes and induce specific clonal selection of these cells.16
This rationalization of the sensitisation process has led to the development of additional assays, addressing the identified gaps in the sensitisation testing strategy.16
The present work focuses on the key event 2, the reaction of the keratinocytes to their exposure to the hapten-protein conjugate. Currently, one OECD accepted method, the ARE-Nrf2 Luciferase Test17 is available for the evaluation of this specific step of the skin sensitisation and has been demonstrated to be applicable to different types of chemicals.18-20 However, this test is based on a cell culture test system, that limits the range of testable concentrations for lipophilic substances that require the use of organic solvents. Moreover, the testing of complex mixtures such as botanical extracts, although possible, was shown to be difficult, mainly due to solubilization issues and to interference related to cytotoxic effects from the organic solvent or from a component of the mixture.21
In parallel to the development and validation of the ARE-Nrf2 Luciferase Test, the NCTC2544-IL18 assay was developed.22-24 This assay is based on the specific release of IL-18 from the keratinocyte cell line NCTC2544 upon exposure to a skin sensitiser and can differentiate skin sensitisers from non-sensitisers. Further investigation revealed that this specific inflammatory reaction of the keratinocytes is also present in other keratinocytes cells such as primary cells. In consequence, recent developments of the assay lead to the transfer of the concept to reconstructed human epidermises, produced from primary keratinocytes, identified generically as the rhe/IL-18 assay.
Several types of reconstructed Human epidermis (rhe) have been evaluated for their suitability for the IL-18 assay.25,26 The assay has been proven to be reliable when performed on 3 commercially available rhe models and one from the Amsterdam university (EpiDERMTM, EpiCSTM, RHETM and VUMC-EE respectively). Although these reconstructed human epidermises were at first designed for skin corrosion and irritation testing, it is not surprising that they respond to skin sensitisers by releasing specific pro-inflammatory cytokines, since the majority of skin sensitisers are also skin irritants.27-31
This aspect complicates the identification of sensitisers that are not irritant and irritant, as the tissue challenge used in the test concept elicits both responses in proportion to the concentration of the tested substance applied. The key being to find the point at which the IL-18 release, specific to the sensitisation pathway, is sufficiently stimulated to be distinguishable from the interference of the irritation response. Although the same two variables, cell viability and IL-18 release, are measured in all the different models of reconstructed epidermis, there are significant differences in the scales of IL-18 release and cell sensitivity between the models reconstructed epidermis. One of the major challenges is thus the optimisation of the interpretation of the observed data for each of the assay systems in a test system specific prediction model that can be subjected to empirical validation.
In this study, we present data obtained from the rhe/IL-18 assay performed using the EpiCSTM reconstructed Human epidermis. 20 reference substances were tested, and the results were interpreted for the classification of the substances as sensitisers or non-sensitisers following a newly developed prediction model. The prediction results were also compared to those obtained using the prediction models published by Gibbs et al.,25 The prediction performances were calculated for all prediction models and compared in order to determine an optimized prediction model applicable for the assay on EpiCSTM reconstructed Human epidermis.
Chemicals and reagents
Phosphate buffered saline (PBS), acetone, olive oil, sulphuric acid, 3,3’,5,5’-tetramethylbenzidine (TMB), sodium carbonate, sodium bicarbonate, bovine albumin serum (BSA), Methyl Thiazolyldiphenyl-Tetrazolium bromide (MTT), isopropanol and tween 20 were purchased from Sigma (St. Louis, MO, USA). Recombinant IL-18, anti-IL-18 antibody and biotinyled antibody were obtained from R&D system (Minneapolis, MN, USA). Streptavidin-HRP was purchased from Thermo-scientific (Waltham, MA, USA). All test items were purchased from Sigma (St. Louis, MO, USA), except for Formaldehyde, obtained from Merck (Darmstadt, Germany).
Reconstructed Human Epidermis and specific culture media
The EpiCSTM reconstructed tissues were produced by CellSystems (Troisdorf, Germany) together with adapted maintenance and assay media. The EpiCSTM models consist of human keratinocytes seeded on a Transwell insert and cultivated as stratified and differentiated 3D culture. The models were delivered on day 17 and used on days 18 to 19 after seeding. During this period, the epidermal models were maintained according to the supplier’s instructions at 37°C, 5% CO2 and 95% relative humidity.
Rhe/IL-18 assay
The rhe/IL-18 assay was performed following previously described process Andres et al.26 Briefly, the test systems (reconstructed epidermises) were exposed to the test items, then, after a defined period of exposure, the release of IL-18 cytokine and the viability of the tests systems were evaluated by ELISA and MTT assays, respectively. The results from both tests were combined to classify the test items following predefined prediction models described in this paper.
Test items
20 reference substances were selected based on their sensitising category, the details are presented on Table 1.
|
Name |
CAS |
Category |
Reference |
Vehicle |
|
DNCB |
97-00-7 |
Extreme Sensitiser (Cat. 1) |
Basketter et al.34 |
AOO |
|
Oxazolone |
15646-46-5 |
Extreme Sensitiser (Cat. 1) |
Gibbs et al.25 |
AOO |
|
PPD |
106-50-3 |
Extreme Sensitiser (Cat. 1) |
Basketter et al.34 |
AOO |
|
Formaldehyde |
50-00-0 |
Strong Sensitiser (Cat. 2) |
Basketter et al.34 |
PBS |
|
Isoeugenol |
97-54-1 |
Strong Sensitiser (Cat. 2) |
Basketter et al.34 |
AOO |
|
Cinnamaldehyde |
104-55-2 |
Strong Sensitiser (Cat. 2) |
Basketter et al.34 |
AOO |
|
Chlorpromazine |
69-09-0 |
Moderate Sensitiser (Cat. 3) |
Basketter et al.34 |
PBS |
|
Citral |
5392-40-5 |
Moderate Sensitiser (Cat. 3) |
Basketter et al.34 |
AOO |
|
2-MBT |
149-30-4 |
Moderate Sensitiser (Cat. 3) |
Basketter et al.34 |
AOO |
|
Cinnamic alcohol |
104-54-1 |
Moderate Sensitiser (Cat. 3) |
Basketter et al.34 |
AOO |
|
Eugenol |
97-53-0 |
Moderate Sensitiser (Cat. 3) |
Basketter et al.34 |
AOO |
|
Benzocaine |
94-09-7 |
Weak Sensitiser (Cat. 4) |
Basketter et al.34 |
AOO |
|
HCA |
101-86-0 |
Very weak Sensitiser (Cat. 5) |
Basketter et al.34 |
AOO |
|
Methyl salicylate |
119-36-8 |
Very weak Sensitiser (Cat. 5) |
Basketter et al.34 |
AOO |
|
Lactic Acid |
50-21-5 |
Non-Sensitiser (Cat. 6) |
Basketter et al.34 |
PBS |
|
Tween 20 |
9005-64-5 |
Non-Sensitiser (Cat. 6) |
Gibbs et al.25 |
AOO |
|
Phenol |
108-95-2 |
Non-Sensitiser (Cat. 6) |
Basketter et al.34 |
AOO |
|
Salicylic Acid |
69-72-7 |
Non-Sensitiser (Cat. 6) |
Basketter et al.34 |
AOO |
|
Chlorobenzene |
108-90-7 |
Non-Sensitiser (Cat. 6) |
Gibbs et al.25 |
AOO |
|
Octanoic acid |
124-07-2 |
Non-Sensitiser (Cat. 6) |
Basketter et al.34 |
AOO |
Table 1 Tested chemicals and vehicles
AOO, acetone,oliveoil (4:1); PBS, Phosphate Buffer Saline; DNCB, 2,4-Dinitrochlorobenzene; PPD, p-Phenylenediamine; 2-MBT, 2-mercaptobenzothiazole; HCA , Hexylcinnamaldehyde. Categories from LLNA assay were translated into potency categories following this scheme, Extreme (LLNA category 1), Strong (LLNA category 2), Moderate (LLNA category 3), Weak (LLNA category 4), Very weak (LLNA category 5) and non-sensitiser (LLNA category 6)
Solubility test and dose range selection
Most of the tested substances were well established sensitisation controls, therefore the EC50 of many of these compounds had already been characterised25 and an adapted dose range, centred on the expected EC50 was selected as first intention dose range in our study. Substances with unknown EC50 or solubility, were tested for their solubility prior to starting the rhe/IL-18 assay with the initial default starting concentration of 200mg/ml in Acetone-Olive Oil (AOO, 4:1 acetone/olive oil) as the first-intention solvent. In the case of insolubility in this solvent, PBS was used. If the substance demonstrated poor solubility when attempted at 200mg/ml, the solubility was verified at 100mg/ml or lower if needed.
Chemical exposure
After 18 to 24 h of equilibration time in culture medium, the epidermis models were exposed to 36µl/cm² of a range of 8 concentrations of the test chemicals typically ranging from 200mg/ml maximum to 1.6mg/ml using a filter paper disc, as described in previous studies.26 After 24 hours of exposure at 37°C, 5% CO2 and 95% relative humidity, the viability of the epidermises was evaluated by MTT assay and the culture medium was sampled for IL-18 ELISA.
If the solubility of the test item did not permit obtaining a 200mg/ml concentration, or if the substance was known to induce high cytotoxicity at such concentration, the dose was replaced by the maximum concentration obtained during solubility testing or to an acceptable concentration in terms of cytotoxicity, and the 7 other concentrations were adapted to cover the range from this maximum to 1/27 of the initial concentration.
Viability assay
After the exposure period, the tissues were thoroughly washed with phosphate buffer and exposed to MTT at 1mg/ml for 3 hours at 37°C, 5% CO2 and 95% relative humidity. Subsequently, the MTT solution was removed and the formazan produced in the tissues was extracted using pure isopropanol for 2 hours under slight agitation. After extraction, the Optical Densities of the isopropanolic extracts were measured by spectrophotometry at 570nm and corrected by subtracting the blank OD.
The viabilities of the epidermis were then calculated as a ratio of the corrected optical densities of the sample over the negative control.
With:
NCOD=OD negative control
PFOD =OD treated tissue with the test item
The EC50, concentration dose of test item producing a 50% decrease in the viability of the epidermis, was calculated following the INVITTOX method:
With:
X=the % of viability immediately higher than 50 %
C=test item concentration leading to X viability
X1=the % of viability immediately lower than 50 %
C1= test item concentration leading to X1 viability
ELISA assay
In-house ELISA kits were prepared as previously described.26 Briefly, 96 well-plates were coated with anti-IL-18 antibody. Following a wash step, the 96 well-plates were blocked with BSA and subjected to another wash step. Subsequently, the sample of medium collected after the 24 hours of test item exposure or standard concentrations of human IL-18 (standard curve) were dispensed in duplicate wells and the plates incubated at RT for 2h.
After another washing step, biotinylated detection antibody was added in each well and the plates were incubated for 1hour at RT. The plates were subsequently washed and Streptavidin-HRP added. After incubation, the plates were washed and TMB was added. The reaction was stopped by the addition of stopping solution (sulphuric acid 1M), leading to the formation of a yellow coloured solution of which the intensity of coloration is proportional to the concentration of IL-18.
The OD values were read by a spectrophotometer (Spectramax, Molecular Devices) at 450nm and the IL-18 concentrations calculated in pg/ml from the 4 parameters logistic calibration curve constructed from at least 7 calibration standards between 5 and 250 pg/ml analysed concurrently with each batch of samples. The results were accepted if the recalculated concentrations of at least 6 of the 7 calibration standards in the calibration curve were within 30% of their nominal values at concentrations above and within the LLOQ. No extrapolation of results was permitted beyond the nominal upper and lower concentrations of the calibration standards.
Sensitisation factor calculation
The IL-18 concentrations were converted to a Sensitisation Factor (SF) which is the ratio between the IL-18 concentration released from chemical-treated epidermises and the chemical concentration applied to induce this IL-18 release:
Data analysis
The experiments were performed in duplicate (two independent experimental runs) and different batches of epidermises were used for each run. The results obtained from each run are presented in Table 4.
The data were interpreted using a newly developed prediction model based on the Sensitisation factor (SF), referred hereinafter as prediction model “SF 5-50”.The SF 5-50 prediction model considers the maximum sensitisation factor (SF) calculated for an epidermis exhibiting a residual viability between 50% and 5%. The cut-off value for SF for the classification of the chemical was fixed at 1. For a SF>1, the substance is considered as a sensitiser, and for SF≤1, the substance is considered as a non-sensitiser. The prediction model was tested on two independent runs consisting of 2 epidermis units treated per substance per run. In case of incoherent results between the two runs, the final classification was considered as positive. If the substance did not induce at least 50% of cytotoxicity at any of the tested concentrations, it was considered as non-sensitiser. If the test item induced a very rapid and severe drop in viability and no tested concentration produced an experimentally observed viability between 50 and 5 %, the SF was calculated from the first concentration exhibiting a viability lower than 5%.
Performances calculation
The performance statistics of the results obtained were evaluated against the in vivo reference results25,34 by using a confusion matrix. The in vivo classifications were considered as the “true conditions” and the classifications obtained using the rhe/IL-18 assay with the SF 5-50 model were considered as the “predicted conditions” (Table 2).
|
|
True conditions |
||
|
Y |
N |
||
|
Predicted conditions |
Y |
“Positive” |
“False positive” |
|
N |
“False negative” |
“Negative” |
|
Table 2 Confusion matrix
Y, Substances classified as sensitising; N, Substances classified as non-sensitising
For each lot of epidermises used, the basal level of IL-18 released under non-exposed (exposed to air) and solvent-exposed (Acetone-Olive Oil and PBS) conditions was controlled. Table 3 shows the mean IL-18 concentration obtained over 3 epidermises for each batch of epidermis used.
|
Epidermis batch # |
NE |
AOO |
PBS |
|
1 |
22.13 |
27.00 |
18.66 |
|
2 |
56.87 |
65.62 |
38.84 |
|
3 |
8.46 |
17.39 |
5.81 |
|
4 |
9.66 |
8.01 |
6.13 |
|
5 |
4.92 |
5.58 |
11.61 |
|
6 |
5.20 |
5.23 |
4.82 |
|
7 |
<LLOQ |
6.30 |
9.70 |
|
8 |
7.53 |
13.28 |
15.90 |
|
9 |
6.66 |
6.25 |
6.84 |
|
10 |
15.02 |
12.88 |
39.88 |
|
Average |
15.16 |
16.75 |
15.82 |
|
S.D. |
16.58 |
18.48 |
13.20 |
|
n |
9 |
10 |
10 |
Table 3 IL-18 release from non-exposed and solvent-exposed epidermises
NE, Non-exposed; AOO, acetone, olive oil (4:1); PBS, Phosphate Buffer Saline. All data are in pg/ml of IL-18. Quantification of IL-18 release from non-exposed epidermises in run 7 was below the lower limit of quantification (not taken into account for calculation)
A total of ten batches of epidermises (EpiCSTM models from Cell Systems) were tested. For the non-exposed condition, the average release of IL-18was 15.16pg/ml (SD: 16.58). Acetone-Olive Oil and PBS treated epidermises exhibited average IL-18 releases of 16.75pg/ml (SD: 18.48) and 15.82pg/ml (SD: 13.20) respectively. No significant difference of IL-18 release between non-exposed, and solvent-treated epidermises was detected, the IL-18 values and their standard deviation were similar.
Stimulation index and EC50 calculations
The substances presented in Table 1 were tested in two independent runs and, for each run, the releases of IL-18 and the final viability were evaluated. The IL-18 release and final viability for the dose ranges of each substance are presented in Figure 1a and Figure 1b.
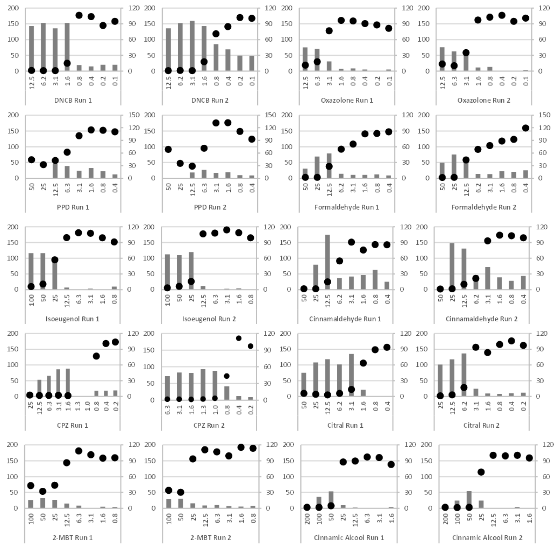
Figure 1a Dose-response curve of IL-18 release and residual viability.
DNCB, 2,4-Dinitrochlorobenzene; PPD, p-Phenylenediamine; CPZ, Chlorpromazine; 2-MBT, 2-mercaptobenzothiazole. EpiCSTM models were exposed to increasing concentrations of test items (mg/ml). Each substance was tested in two independent runs (graphs provided side by side). Black circles, relative viability data, right scale in % of control viability. Grey bars, IL-18 concentrations measured, left scale in pg/ml.
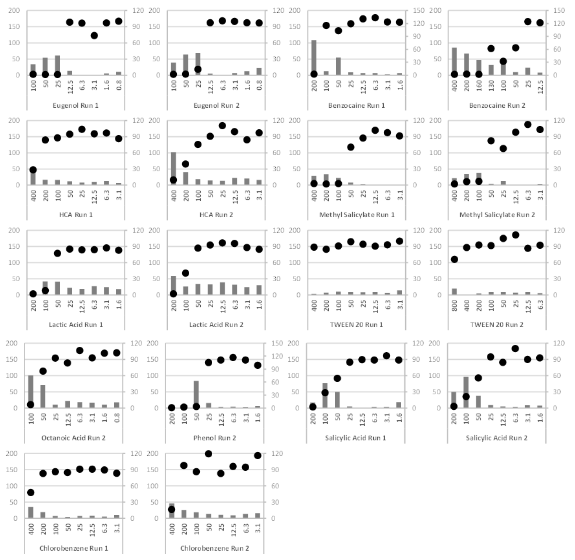
Figure 1b Dose-response curve of IL-18 release and residual viability.
HCA, Hexyl cinnamaldehyde. EpiCSTM models were exposed to increasing concentrations of test items (mg/ml). Each substance was tested in two independent runs (graphs provided side by side, excepted for Octanoic Acid and Phenol, one run accepted). Black circles, relative viability data, right scale in % of control viability. Grey bars, IL-18 concentrations measured, left scale in pg/ml.
The results presented in Figure 1a and Figure 1b were used to calculate the stimulation factor (SF 5-50) as defined previously. The maximum relevant IL-18 concentration and the corresponding test item concentration are presented in Table 4.
|
Name |
Run # |
EC50(mg/ml) |
Test item concentration (mg/ml) |
IL-18 (pg/ml) |
SF 5-50 |
|
DNCB |
1 |
1.29 |
1.57 |
153.18 |
98.19 |
|
2 |
1.12 |
1.57 |
143.17 |
91.78 |
|
|
Oxazolone |
1 |
4.56 |
12.5 |
75.55 |
6.04 |
|
2 |
2.74 |
12.5 |
76.25 |
6.10 |
|
|
PPD |
1 |
10.06 |
12.5 |
45.21 |
3.62 |
|
2 |
9.37 |
6.25 |
26.12 |
4.18 |
|
|
Formaldehyde |
1 |
7.23 |
12.5 |
78.56 |
6.28 |
|
2 |
10.89 |
12.5 |
66.99 |
5.36 |
|
|
Isoeugenol |
1 |
28.77 |
25.0 |
102.64 |
4.11 |
|
2 |
20.28 |
25.0 |
119.55 |
4.78 |
|
|
Cinnamaldehyde |
1 |
7 |
12.5 |
175.24 |
14.02 |
|
2 |
4.98 |
12.5 |
131.05 |
10.48 |
|
|
CPZ |
1 |
1.06 |
1.57 |
87.68 |
56.21 |
|
2 |
0.74 |
1 |
88.09 |
88.09 |
|
|
Citral |
1 |
1.97 |
3.13 |
134.79 |
43.20 |
|
2 |
3.89 |
6.25 |
136.56 |
21.85 |
|
|
2-MBT |
1 |
23.21 |
50.0 |
32.34 |
0.65 |
|
2 |
42.21 |
50.0 |
30.38 |
0.61 |
|
|
Cinnamic Alcohol |
1 |
36.38 |
50.0 |
53.59 |
1.07 |
|
2 |
31.99 |
50.0 |
54.76 |
1.10 |
|
|
Eugenol |
1 |
18.72 |
25.0 |
60.86 |
2.43 |
|
2 |
19.36 |
25.0 |
68.75 |
2.75 |
|
|
Benzocaine |
1 |
158.02 |
50.0 |
54.85 |
1.10 |
|
2 |
135.95 |
25.0 |
52.66 |
2.11 |
|
|
HCA |
1 |
321.73 |
400 |
48.23 |
0.12 |
|
2 |
169.73 |
400 |
102.03 |
0.26 |
|
|
Methyl Salicylate |
1 |
65.07 |
100 |
22.64 |
0.23 |
|
2 |
71.5 |
100 |
37.47 |
0.37 |
|
|
Lactic Acid |
1 |
69.55 |
100 |
41.82 |
0.42 |
|
2 |
89.74 |
100 |
26.41 |
0.26 |
|
|
Tween 20 |
1 |
NA |
400 |
3.42 |
0.01 |
|
2 |
NA |
800 |
19.45 |
0.02 |
|
|
Phenol |
1 |
ND |
ND |
ND |
ND |
|
2 |
38.62 |
50.0 |
83.78 |
1.68 |
|
|
Salicylic Acid |
1 |
59.84 |
100 |
77.04 |
0.77 |
|
2 |
58.63 |
100 |
96.6 |
0.97 |
|
|
Chlorobenzene |
1 |
388.62 |
400 |
35.21 |
0.09 |
|
2 |
249.53 |
400 |
46.28 |
0.12 |
|
|
Octanoic Acid |
1 |
ND |
ND |
ND |
ND |
|
2 |
65.03 |
100 |
100.29 |
1.00 |
Table 4 EC50 and maximum Sensitisation factor for each substance
DNCB, 2,4-Dinitrochlorobenzene; PPD, p-Phenylenediamine; CPZ, Chlorpromazine; 2-MBT, 2-mercaptobenzothiazole; HCA, Hexylcinnamaldehyde. For each chemical, the results and calculations from two experimental runs are presented. Test item concentration is the test item concentration corresponding to the maximum IL-18 concentration used for the Sensitisation Factors (SF) calculation. ND, not-determined, Only one run performed for the testing of Phenol and Octanoic acid
The sensitisation factors were interpreted following the SF 5-50 prediction model, in order to determine the sensitising/non-sensitising status of the chemical.The interpretations are presented in Table 7.
Test performance statistics
The classifications obtained in Table 7 were used to calculate the test prediction performance, according to the prediction model used. The results of the confusion matrix are presented in Table 8.
|
|
RHETM |
EpiCSTM |
|
N |
17 |
10 |
|
Non-exposed |
56.5±23.7 |
15.2 ±16.6 |
|
AOO |
97.8±55.0 |
16.7±18.5 |
|
PBS |
90.7±28.7 |
15.8±13.2 |
Table 5 Comparison of the IL-18 release from non-exposed and solvent exposed RHETM model (Historical data) and EpiCSTM model (current data)
RHETM: Epidermises model from SkinEthic laboratories; EpiCSTM: Epidermises models from CellSystems laboratories. N is the total number of epidermises batches used for the calculation the averages. AOO = acetone: olive oil (4:1); PBS: Phosphate Buffer Saline. Data obtained for the RHE were extracted from Andres et al. 2017
|
Nom |
Run # |
SI calculation |
SFxmax |
||
|
Max SI |
EC50 (mg/ml) |
Test item concentration (mg/ml) |
Max SFx |
||
|
DNCB |
1 |
5.7 |
1.29 |
1.57 |
580.63 |
|
2 |
2.2 |
1.12 |
1.57 |
59.86 |
|
|
Oxazolone |
1 |
12.6 |
4.56 |
6.25 |
131.58 |
|
2 |
12.0 |
2.74 |
3.13 |
302.08 |
|
|
PPD |
1 |
2.6 |
10.06 |
6.25 |
10.96 |
|
2 |
3.3 |
9.37 |
6.25 |
21.73 |
|
|
Formaldehyde |
1 |
8.1 |
7.23 |
12.5 |
34.54 |
|
2 |
4.2 |
10.89 |
12.5 |
13.37 |
|
|
Isoeugenol |
1 |
20.9 |
28.77 |
25.0 |
58.42 |
|
2 |
22.8 |
20.28 |
25.0 |
148.78 |
|
|
Cinnamaldehyde |
1 |
6.5 |
7.00 |
12.5 |
32.66 |
|
2 |
2.0 |
4.98 |
3.13 |
3.55 |
|
|
CPZ |
1 |
3.3 |
1.06 |
1.57 |
1166.32 |
|
2 |
6.8 |
0.74 |
1.00 |
1369.31 |
|
|
Citral |
1 |
21.6 |
1.97 |
3.13 |
590.38 |
|
2 |
10.6 |
3.89 |
6.25 |
215.41 |
|
|
2-MBT |
1 |
5.1 |
23.21 |
25.0 |
6.49 |
|
2 |
2.3 |
42.21 |
50.0 |
2.73 |
|
|
Cinnamic Alcohol |
1 |
1.8 |
36.38 |
50.0 |
25.78 |
|
2 |
4.8 |
31.99 |
50.0 |
15.05 |
|
|
Eugenol |
1 |
0.5 |
18.72 |
25.0 |
13.52 |
|
2 |
1.0 |
19.36 |
25.0 |
6.69 |
|
|
Benzocaine |
1 |
9.4 |
158.02 |
200 |
6.21 |
|
2 |
6.6 |
135.95 |
25.0 |
13.60 |
|
|
HCA |
1 |
7.7 |
321.73 |
400 |
1.41 |
|
2 |
7.9 |
169.73 |
6.25 |
1.46 |
|
|
Methyl Salicylate |
1 |
1.4 |
65.07 |
100 |
3.57 |
|
2 |
2.4 |
71.50 |
100 |
9.74 |
|
|
Lactic Acid |
1 |
2.2 |
69.55 |
50.0 |
0.33 |
|
2 |
1.0 |
89.74 |
3.13 |
0.35 |
|
|
Tween 20 |
1 |
0.8 |
NA |
100 |
0.04 |
|
2 |
2.4 |
NA |
12.5 |
0.34 |
|
|
Phenol |
1 |
ND |
ND |
ND |
ND |
|
2 |
1.2 |
38.62 |
50.0 |
20.86 |
|
|
Salicylic Acid |
1 |
12.2 |
59.84 |
3.13 |
11.84 |
|
2 |
7.3 |
58.63 |
50.0 |
3.13 |
|
|
Chlorobenzene |
1 |
5.6 |
388.62 |
6.25 |
1.58 |
|
2 |
3.6 |
249.53 |
400 |
0.65 |
|
|
Octanoic Acid |
1 |
ND |
ND |
ND |
ND |
|
2 |
7.8 |
65.03 |
50.0 |
4.43 |
|
Table 6 Maximum relevant Stimulation Index and SFx for each substance
DNCB, 2,4-Dinitrochlorobenzene; PPD, p-Phenylenediamine; CPZ, Chlorpromazine; 2-MBT, 2-mercaptobenzothiazole ; HCA , Hexylcinnamaldehyde. For each chemical, the results and calculations from two experimental runs are presented. Max SI is the maximum Stimulation Index that can be taken into account considering the viability criteria. SFxmax was calculated as described in the discussion. ND, not-determined, Only one run performed for the testing of Phenol and Octanoic acid. NA, not available, No EC50 achieved under the testing conditions
|
Class |
Nom |
Run # |
SF 5-50 |
Sfx |
5x 5-40 |
2x 5-40 |
||||
|
Prediction |
Status |
Prediction |
Status |
Prediction |
Status |
Prediction |
Status |
|||
|
Extreme |
DNCB |
1 |
+ |
TP |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
+ |
- |
+ |
||||||
|
Extreme |
Oxazolone |
1 |
+ |
TP |
+ + |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
+ |
+ |
|||||||
|
Extreme |
PPD |
1 |
+ |
TP |
+ |
TP |
- |
FN |
+ |
TP |
|
2 |
+ |
+ |
- |
+ |
||||||
|
Strong |
Formaldehyde |
1 |
+ |
TP |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
+ |
- |
+ |
||||||
|
Strong |
Isoeugenol |
1 |
+ |
TP |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
+ |
+ |
+ |
||||||
|
Strong |
Cinnamaldehyde |
1 |
+ |
TP |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
- |
- |
+ |
||||||
|
Moderate |
Chlorpromazine |
1 |
+ |
TP |
+ |
TP |
- |
TP |
+ |
TP |
|
2 |
+ |
+ |
+ |
+ |
||||||
|
Moderate |
Citral |
1 |
+ |
TP |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
+ |
+ |
+ |
||||||
|
Moderate |
2-MBT |
1 |
- |
FN |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
- |
- |
- |
+ |
||||||
|
Moderate |
Cinnamic Alcohol |
1 |
+ |
TP |
+ |
TP |
- |
FN |
- |
TP |
|
2 |
+ |
+ |
- |
+ |
||||||
|
Moderate |
Eugenol |
1 |
+ |
TP |
+ |
TP |
- |
FN |
- |
FN |
|
2 |
+ |
+ |
- |
- |
||||||
|
Weak |
Benzocaine |
1 |
+ |
TP |
+ |
TP |
+ |
TP |
+ |
TP |
|
2 |
+ |
+ |
+ |
+ |
||||||
|
Very weak |
HCA |
1 |
- |
FN |
- |
FN |
+ |
TP |
+ |
TP |
|
2 |
- |
- |
+ |
+ |
||||||
|
Very weak |
Methyl Salicylate |
1 |
- |
FN |
- |
TP |
- |
FN |
- |
TP |
|
2 |
- |
+ |
- |
+ |
||||||
|
Non-sensitiser |
Lactic Acid |
1 |
- |
TN |
- |
TN |
- |
TN |
+ |
FP |
|
2 |
- |
- |
- |
- |
||||||
|
Non-sensitiser |
Tween 20 |
1 |
- |
TN |
- |
TN |
- |
TN |
- |
FP |
|
2 |
- |
- |
- |
+ |
||||||
|
Non-sensitiser |
Phenol |
1 |
ND |
FP |
ND |
FP |
ND |
TN |
ND |
TN |
|
2 |
+ |
+ |
- |
- |
||||||
|
Non-sensitiser |
Salicylic Acid |
1 |
- |
TN |
+ |
FP |
+ |
FP |
+ |
FP |
|
2 |
- |
- |
+ |
+ |
||||||
|
Non-sensitiser |
Chlorobenzene |
1 |
- |
TN |
- |
TN |
+ |
FP |
+ |
FP |
|
2 |
- |
- |
- |
+ |
||||||
|
Non-sensitiser |
Octanoic Acid |
1 |
ND |
TN |
ND |
TN |
ND |
FP |
ND |
FP |
|
2 |
- |
- |
+ |
+ |
||||||
Table 7 Prediction classifications obtained using Stimulation Index based prediction models
DNCB, 2,4-Dinitrochlorobenzene; PPD, p-Phenylenediamine; 2-MBT, 2-MercaptoBenzoThiazole; HCA, Hexylcinnamicaldehyde; ND, non-determined; Only one run performed for the testing of Phenol and Octanoic acid; TP, True Positive, TN, True negative; FP, false positive; FN, False negative. Different classification over the two runs for one chemical are grey-shaded. Status is the comparison between the prediction of the test and the reference classification from LLNA assay, False positive (FP) and False negative (FN) prediction are bold and italic
|
PM |
Sensitivity |
Specificity |
Accuracy |
|
5x 5-40 |
71% |
50% |
65% |
|
2x 5-40 |
93% |
17% |
70% |
|
SFx |
93% |
67% |
85% |
|
SF 5-50 |
79% |
83% |
80% |
Table 8 Prediction performances of the 4 Prediction Models used
PM, Prediction Model. Performance statistics calculated with respect to LLNA based classifications as reference
While many studies have been conducted for the development and evaluation of the rhe/IL-18 assay, there are still many uncertainties regarding the most appropriate prediction algorithm to apply for distinguishing sensitisers from non-sensitisers and irritants in each model, and which results to consider as gold standard for the evaluation of their performance.26
The aim of this study was the evaluation of the prediction capacities of the rhe/IL-18 assay using the EpiCSTM reconstructed epidermis model by applying a prediction model based on the relationship between the actual amount of IL-18 released and the test item concentration needed to induce this release.
20 well characterised references chemicals covering all ranges of sensitiser potency were chosen and tested on EpiCSTM epidermises for the initial evaluation of the assay with respect to the testing of pure chemicals. The extension of the applicability domain of the assay with respect to other types of substances, such as mixtures or botanical extracts, will be undertaken in future work.
Previous work performed using Skin Ethic RHETM reconstructed epidermis model underlined the importance of the consistency of the epidermis inflammatory state.26 Indeed, a too high or too variable basal level of IL-18 release reduces the sensitivity of the effective detection of IL-18 release variations and thus, leads to poor prediction performance of the assay, regardless of any modulations of the prediction model.
The CellSystems EpiCSTM epidermis model exhibits significantly lower basal levels of IL-18 release and significantly lower variability between the different batches compared to the results obtained with the Skin Ethic RHETM Model (Table 5). This may be due to the intrinsic properties of the epidermis model. Recent investigations conducted on the RHETM model indicated that the variability may be due to the characteristics of the primary cells used for the epidermis production. The RHETM reconstructed epidermises are cultured from primary keratinocytes extracted from young human foreskin and adult human abdominal surgery, whereas the EpiCSTM reconstructed epidermises are all cultured only from primary keratinocytes extracted from foreskin. It is likely that differences in terms of age of the donor and body area origin may impact the overall inflammation status of the epidermises reconstructed from these cells and that the mixed origin of the primary keratinocytes would results in higher variability of the basal IL-18 release characteristics of the model.
In addition to the basal level of IL-18, particular attention was paid to the effect of classic solvents (AOO, PBS) on the IL-18 release. While these solvents induced very variable and significant releases of IL-18 in RHETM model,26 neither of them induced any significant increase of the release of IL-18 on the EpiCSTM model when compared to the non-exposed epidermises (Table 5). This is a clear advantage, considering the interest of having the possibility of using either an aqueous solvent and a lipophilic solvent, although most of the substances tested in the work reported in this paper were solubilized in AOO (17/20) which is the first-choice solvent for such testing. Moreover, the mean basal IL-18 levels observed in the EpiCSTM model during this study also closely resembled those measured in our laboratories in 2016 (17.02pg/ml)26 showing the reproducibility of these data over time.
The rhe/IL-18 assay monitors two related measurable biological phenomena, IL-18 release and viability, to assess the potential sensitisation effect. The algorithm to calculate the sensitisation factor SF proposed for the evaluation of the data empirically integrates both phenomena in the evaluation by identifying the maximum IL-18 concentration in the 5% to 50% viability range for the calculation of SF by division of the IL-18 concentration with the test item concentration. This factor thus empirically takes into account the effects of the IL-18 release, the viability and the potency.
However, the individual, point by point evaluation of the applied concentrations can be complicated if the concentration range is too wide and the change in viability is too abrupt between two concentrations, or if the experimental results do not include the change in viability required by the algorithm. It is evidently very difficult to predict the appropriate concentration range for unknown test substances that have not yet been characterised. Ideally the concentration ranges should be refocussed around the concentration of test item at which the viability starts to decrease, however, practically it becomes too onerous and expensive to repeat the experiment with a full range of concentration levels and controls.
In the current dataset, several of the substances produced changes in viability between concentrations that were very abrupt and did not provide a distinct point between 5% and 50%. However, the rhe/IL-18 assay has the advantage of modelling a significant series of concentrations and this permits the evaluation of the data as pseudo continuous by differential analysis. Differential analysis compensates for the apparent discontinuities in the viability and IL-18 release data and permits the detection of changes over the entire range of the results.
The two monitored effects of sensitisation, increase in IL-18 concentration and the decrease in viability between the concentrations, can be evaluated in combination as a function of the changes in test item concentration, expressed as a sensitisation factor SFx as follows:
Where;
The difference in IL-18 concentration index, [IL18i], is calculated as the increase in IL-18 concentration index between one concentration and the next higher concentration. Cx is the concentration of test item, where x=0 is the lowest test item concentration applied to the tissues. The calculation therefore begins from the second highest test item concentration (x=1).
The IL-18 concentration index, [IL18i], is defined as the measured IL-18 concentration at each test item concentration, [IL18]Cx, corrected by measured IL-18 release of the appropriate solvent control for the batch of epidermises, [IL18]S, normalised as a fraction of the IL-18 release concentration of the appropriate solvent control for the specific run.
The basal IL-18 releaseis considered to be an intrinsic property of the batch of epidermises, variable from batch to batch, but assumed to be consistent within the batch. The IL-18 release measured in the solvent control would thus be representative of the intrinsic basal IL-18 release under the conditions of the particular solvent. This definition makes it possible to apply the same model factor to any reconstructed tissue model to normalise the IL-18 response to the specific tissue model performance and to correct for between batch variability.
The viability, as determined in the assay, is already a fraction normalised with respect to the viability measured in untreated tissue in the same batch of tissue, it is therefore also an intrinsic characteristic of the particular batch of tissue which corrects for batch to batch variability. Consequently, the within run differential can be used directly to characterise changes from one treatment condition to the next in the proposed model.
The change in concentration, [C], is calculated as the increase in concentration between one concentration and the next highest concentration. The calculation starts from the lowest applied concentration upwards.
When applied to the data the Sensitisation Factor, SFx, tends to give distinct maxima when the decrease in viability and the increase in IL-18 concentration tend to be maximised in coordination. This best represents the sensitisation response of the rhe/IL-18 assay. When plotting the Sensitisation Factor, SFx, against the test item concentrations, sensitising test item substances, such as eugenol, tend to produce a pronounced peak, whereas non-sensitising test substances, such as TWEEN, tend to show no or very low responses by comparison (Figure 2).
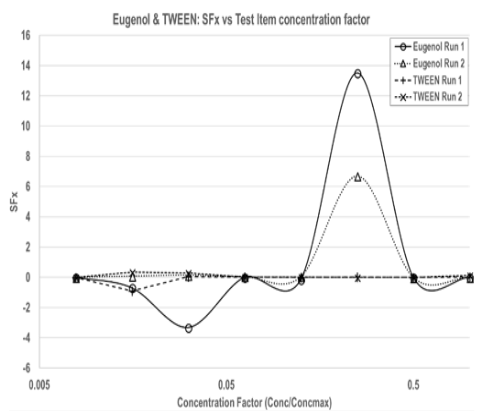
Figure 2 SFx calculated from Eugenol and TWEEN data.
Calculated SFx is plotted against the test item concentration factor (Conc./Conc.max) on a logarithmic scale to permit direct comparisons between the different test substances that were tested with different concentration ranges. (Eugenol: 0.79–100mg/ml; TWEEN: 3.13 – 400 & 6.25– 800mg/ml.
The confusion matrix was used to calculate the 3 main indicators of prediction performance, sensitivity, specificity and accuracy, which are respectively the ratio of success for the prediction of positive substances, negative substances and of all types of substances.
The amplitude of the maximum response can be used to distinguish sensitising test item substances from non-sensitising test item substances and the point on the ordinal axis at which the peak maximum appears related to the concentration of test item at which the sensitisation effect is at its maximum.
The Table 6 summarises the SFx maxima observed for the reference substances subjected to the rhe/IL-18 test in this paper. The cut-off value for SFx for the classification of the chemical was empirically fixed at 4.5. For a SFx>4.5, the substance was considered as a sensitiser, and for SF≤4.5, the substance was considered as a non-sensitiser. The prediction results are presented in Table 7. By virtue of its intrinsic normalisation of both IL-18 and viability responses, this model should prove to be applicable to all tissue models on which the rhe/IL-18 assay is performed, although the cut-off values will be different from one tissue model to the next and should be determined within the context of each laboratory.
During this study, we also applied two prediction models described by Gibbs et al.,24 to compare prediction performances obtained by the SF 5-50and SFx prediction models to other PMs.
The two prediction models described by Gibbs use the stimulation index (SI), which is the ratio between the IL-18 concentration released from chemical-treated epidermises and from solvent-treated epidermises. It represents the variation of released IL-18 due to the treatment applied to the epidermises:
The Stimulation Index was used in the two prediction models (PM) for the interpretation of the results, PM “5x 5-40”: cut-off of 5-fold increase of IL-18 release with respect to the solvent control and with viability in the range of ≥5% and ≤40%. This prediction model was suggested by Gibbs et al. 201324 for the interpretation of the rhe/IL-18 assay data. We also applied a second prediction model: PM “2x 5-40”: cut-off of 2-fold increase of IL-18 release compared to the solvent control and viability ≥5% and ≤40%, in order to test whether an optimization of the prediction performance could be achieved.
In all the applied prediction models, the final classification was considered as positive for the substances with different classification result between the two experimental runs, adopting a worst-case scenario thereby avoiding false negative prediction. These chemicals would have needed a third run, and this would be the strategy for future routine testing.
For each substance, the maximum relevant SI and the SFxare presented in Table 6 and the prediction results are presented in Table 7.
Finally, the prediction performance statistics were calculated as described previously and compared to those obtained with the SF 5-50 prediction model in Table 8.
The prediction performance statistics obtained by the application of the 4 PMs were compared on the basis of their accuracy and coherence with respect to the classifications assigned by Local Lymph Node Assay.18,34
We obtained comparable results in terms of performance statistics and noticed performances trends for each prediction model. The PM “5x 5-40” had average sensitivity (71%) and specificity (50%), indicating that this model is equally adapted for the detection of positive substances and negatives ones, with a global performance of 65% accuracy. The PM “2x 5-40” had a different pattern of performance with a high sensitivity (93%) and a low specificity (17%) indicating that this prediction model is particularly adapted to the detection of positive substances but not negatives ones, with an overall accuracy of 70%.These two prediction models are based on cut-off limits applied to the amplitude of the difference of IL-18 release between the solvent treated and test item treated epidermises.
However, it is apparent that the data interpretation using the Stimulation Index is sensitive to small variations in terms of biological activity. For instance, an IL-18 release of 30pg/ml would be considered as positive if the basal release of IL-18 was 15 pg/ml and negative if the basal release was 17pg/ml, although there is no biological difference between the two situations. Moreover, the dose of test item needed to induce a significant release of IL-18 is not taken into account in this analysis: a release of 30pg/ml of IL-18 for a test item dose of 1mg/ml would be considered as positive if the basal release of IL-18 was 15pg/ml, whereas the same level of IL-18 release will be considered equivalently positive if the test item dose needed for such release is 1000mg/ml which does not have the same biological significance. This illustrates the difficulty to achieve a prediction model well balanced between its detection of positive and negative substances, especially if it is highly dependent of a variable parameter.
Over the 10 runs performed on different batches, the EpiCSTM epidermises system exhibited a relatively stable basal level of IL-18 release (15.6+/- 16.6pg/ml, Table 5). The variability of which we consider insignificant, in terms of biological effect. Even in the presence of the solvents there was no significant induction of IL-18 release.
Therefore, we tested here the application of a novel prediction model, without normalisation by the basal level of IL-18, taking into account the dose needed to induce an IL-18 release (herein referred to as Prediction model “SF 5-50”)as an appropriate approach for the evaluation of sensitisation with EpiCSTM model. Using this alternative prediction model, the prediction performance was better (79 % sensitivity, 83% specificity, 80% accuracy) than that achieved with the previously published prediction models based on the SI, especially with respect to the correct detection of negative substances, thereby diminishing high false positive classification rates.
Considering the two independent experiments for each tested substance, the IL-18 release results exhibited a between run reproducibility that is not reflected in the related SI values calculation (Table 4). This supports the choice of working directly with the real concentration of IL-18 rather than considering the Stimulation Factor.
Further reflection on the optimal way to analyse the results lead us to develop the SFx calculation, in order to provide for continuous evaluation over the entire dose and toxicity response ranges. This more elaborate analysis integrates a more complete set of parameters and exhibits a sensitivity weighted pattern of performance (93% sensitivity, 67% specificity and 85% of accuracy). Its overall performance is, as for the SF 5-50 prediction model, slightly more accurate than that achieved with the other published prediction models on this dataset. The SFx model performs better at the detection of positive substances, which make the overall accuracy also higher than that of the SF 5-50 model.
The combination of the increase in IL-18 concentration and the decrease in viability implies that SFx model is not constrained to a specific viability range and manages to detect sensitisation responses at concentrations where the viability has not yet diminished to less than 50%. Thus, the test item concentrations at which the sensitisation response is detected by the SFx model do not coincide completely with those of the SF 5-50 model.
Using this type of prediction model, we consider the accurate quantification of the IL-18 concentration as critical. Several commercially available kits were tested but did not satisfy our needs in terms of quality and sensitivity, therefore we developed an in-house ELISA assay integrating Quality controls, providing quantification of the IL-18 down to 5pg/ml without extrapolation.
Using PM “5x 5-40”, 6/20 substances gave inconsistent sensitisation results over the two batches while only 4/20 for the PM “2x 5-40”, 4/20 for the PM SFx (Table 7) and none for the PM “SF 5-50” (Table 7) (excluding the Phenol and Octanoic acid for which data from only 1 accepted run was available). This could be explained by the fact that the Stimulation Index is highly impacted by slight variations of the IL-18 release basal level, which is not the case for the Stimulation Factor.
Although LLNA data has always been used as gold standard reference for the performance statistics calculation of sensitisation assessment, and that the wide range of data available from this assay still support its use for the evaluation of new assays in the sensitisation field, there is growing doubt as to the relevance of choosing an animal model as reference for a human in vitro assay evaluation (Piersma et al. 2018).35 Considering that one of the most important limits of in vitro assays is that they only address individual mechanistic steps of toxicological pathways, that obliges their inclusion in an integrated testing strategy within the relevant AOP framework, other ways to select or build a more adapted gold standard should be set-up. These initiatives should be based on a better collaboration between the stakeholders involved in the evaluation of products and assays.
Considering that the applicable regulations European Commission, 20037, strongly restrict the performance of in vivo studies for toxicology assessment, it is evident that the amount of in vivo data available for the assessment of the in vitro assay performance will essentially remain static and thus not keep pace with the number and nature of new substances introduced in the cosmetic, chemical, medical devices and dermatology industries. This will challenge the relevancy of in vivo data as reliable reference standard. We previously proposed a synthetic reference composed of all, validated, in vitro assays available, in order to overcome the lack of relevant in vivo data.26 While not yet sufficiently mature for use, this approach of evaluation should be tested in wide ranging studies with the aim of replacing the animal data as the reference standard in the evaluation of in vitro assays.
Today, the very specific nature of in vitro tests implies that in vitro testing plans need to adopt comprehensive Adverse Outcome Pathway (AOP) strategies. This is particularly relevant to the evaluation of a highly complex phenomenon such as the sensitisation. Since the sensitisation AOP is composed of numerous inter-related key biological events (KE), it is of great interest to have accurate and reliable tests addressing each of the identified key event, in order to achieve the most comprehensive results. In this context, the rhe/IL-18 assay, once validated and its applicability domain clearly defined, will enhance the overall evaluation of the skin sensitisation by providing data from topically applied substances. Moreover, the intrinsic characteristics of reconstructed epidermises, such as metabolic activity, provide testing conditions closer to real physiology. It would thus be of interest to assess further enhanced protocols such as repeated exposure or the application of cocultures integrating cells of particular interest such as dendritic cells to develop a more accurate testing.36-40
The prediction performance statistics obtained using the prediction models SF 5-50 and SFx approach those of other well-established in vitro assays for sensitisation assessment (Table 9) addressing other key events of the skin sensitisation AOP, although the total number of results used for the performance calculation is still modest when compared to those of the other assays.
|
Name |
Ref |
Sensitisation Key event |
Sensitivity |
Specificity |
Accuracy |
|
DPRA |
OECDf, 2015 |
1 |
80% |
77% |
80% |
|
ARE-Nrf2 Luciferase Test |
OECDd, 2015 |
2 |
78% |
76% |
77% |
|
h-CLAT |
OECDe, 2017 |
3 |
93% |
66% |
85% |
|
U-SensTM |
OECDe, 2017 |
3 |
91% |
65% |
86% |
|
rhe/IL-18 (PM “SF 5-50”) |
- |
2 |
79% |
83% |
80% |
|
rhe/IL-18 (PM “SFx”) |
- |
2 |
93% |
67% |
85% |
Table 9 Performances of existing in vitro sensitisation assays compared to rhe/IL-18 assay
In conclusion, based on the presented results, the rhe/IL-18 assay performed with EpiCSTM epidermises model can be considered as a relevant component of AOP based sensitisation testing when combined with an effective prediction model. The new SF 5-50 and SFx prediction models applied to the data from the rhe/IL-18 assay performed on the EpiCSTM epidermis tissue in this work demonstrate performance characteristics that are comparable with currently accepted in-vitro assays albeit with an as yet modest number of test substances. Whereas performance of the majority of prediction models applied in the context of rhe/IL-18 assays appear to be specific to the intrinsic characteristics of the tissue types on which they were developed, the SFx model abstracts the calculation of the test statistic, the Sensitisation Factor, from the intrinsic particularities of the tissue type, thereby potentially making it universally applicable to any of the tissue models. Future work, focused on the increasing of total number of substances tested, as well as the enlargement of the applicability domain of the assay by testing substances of varied natures, is envisaged to increase the accuracy of the performance evaluation for this assay and the related prediction models.
None.
We declare no conflict of interest.
This research was supported by Pierre Fabre Dermo-Cosmétique.

©2020 Andres, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.