Journal of
eISSN: 2574-9943


Case Series Volume 4 Issue 4
1University of Illinois at Chicago Chicago, USA
2Silver Falls Dermatology Portland, USA
3Accent Dermatology Medford, USA
4Forefront Dermatology Ann Arbor, USA
Correspondence: Lauren Boudreaux, Silver Falls Dermatology, USA
Received: July 06, 2020 | Published: August 21, 2020
Citation: Turowski MBS, Boudreaux L, Klein R, et al. A case series of dupilumab-treated atopic dermatitis resulting in new onset psoriasis. J Dermat Cosmetol . 2020;4(4):83-86. DOI: 10.15406/jdc.2020.04.00158
Dupilumab is the first biologic agent approved for the treatment of moderate-to-severe atopic dermatitis (AD). Although throughout clinical testing the medication only caused minor side-effects, there have been an increasing amount of reports of new onset psoriasis during the course of treatment of AD with Dupilumab. This case series explores the previously reported cases of this novel side effect of Dupilumab and then reports the five novel cases that have been previously unrecorded. The findings support a previously proposed TH-1 overdrive based mechanism for the unusual psoriasis development and serve to urge physicians to monitor patients closely on Dupilmab for this potential side effect.
Psoriasis and Atopic Dermatitis (AD) share a common inflammatory-based mechanisms, but are rooted in unique branches of the cytokine pathway, with psoriasis being driven by Th-1 and Th-17 pathways and AD rooted in the Th-2 pathway. The two diseases also differ morphologically, typically allowing dermatologists to distinguish between the two conditions and appropriately tailor treatment.
Dupilumab is the first biologic agent approved for the treatment of adults with moderate-to-severe AD, targeting the IL-4 receptor α. Its efficacy and safety have been studied repeatedly and only minor side effects have been reported.1–4 As the use of dupilumab expands, there are more case reports suggesting unreported cutaneous side effects, such as new-onset psoriasiform dermatitis.
Several cases describing the development of psoriasiform dermatitis during the use of dupilumab have been published. In the majority of cases, new lesions suspicious for psoriasis were confirmed by biopsy. Patients typically received the standard dosing of dupilumab, with sudden onset of psoriasiform dermatitis that was responsive to classic treatments for psoriasis (Table 1).
|
Patient description |
Time treated AD with dupilumabprior to symptoms |
Location of psoriasiform lesions |
Biopsy results |
Subtype of psoriasis diagnosed |
Treatment choice for psoriform lesions when occurred |
Citation |
|
50 year old female with AD and asthma |
4 months |
Bilateral upper and lower extremities, trunk |
Arm: Psoriasiform hyperplasia with a diminished granular layer and focal collections of neutrophils within parakeratotic scale. There was a brisk perivascular and diffuse dermal infiltrate of neutrophils with admixed histiocytes and occasional eosinophils
Abdomen: Irregular acanthosis, mild spongiosis, and intraepidermal neutrophils forming subcorneal pustules. The granular layer was maintained with focal parakeratosis
|
Erythrodermic psoriasis |
Methotrexate and topical steroids |
3 |
|
40 year old female with AD |
16 weeks |
Trunk and extremities |
Parakeratosis, hyperkeratosis, acanthosis, dilated capillaries, and a lymphocytic infiltrate in the upper dermis |
Guttate psoriasis |
Continued duplimab and added daily topical calcipotriol-betamethasone foam |
2 |
|
55 year old male with AD |
2 months |
Trunk |
Parakeratosis, hyperkeratosis, acanthosis, dilated capillaries and a lymphocytic infiltrate in the upper dermis |
Psoriasis Vulgaris |
Continued dupilumab and added topical steroid |
3 |
|
59 year old female with AD |
4 weeks (second round of treatment, was first discontinued by patient after 36 weeks) |
Upper and lower extremities |
Confluent parakeratosis with absent granular cell layer, regular acanthosis, and thinning of the suprapapillary plates |
Psoriasis Vulgaris |
Discontinued dupilumab and added triamcinolone ointment |
4 |
|
54 year old male with AD |
8 months |
Bilateral upper and lower extremities, chest, back, neck, and abdomen
|
Acute spongiotic dermatitis with lymphocyte exocytosis |
Palmoplantar and erythrodermic psoriasis |
Discontinued dupilumab |
1 |
|
49 year old female with AD |
1.5 years |
upper and lower extremities
|
Scattered, disorganized fingernail pits |
None reported |
Continued dupilumab and added twice-daily clobetasol ointment |
1 |
|
Female in 50s with asthma and AD |
2 months |
Widespread: scalp, trunk and bilateral upper and lower extremities with thick white scale
|
Psoriasiform hyperplasia with a diminished granular layer and focal collections of neutrophils within parakeratotic scale
|
None reported |
Discontinued dupilumab and added methotrexate for several months with return to skin baseline |
5 |
Table 1 Below is a chart summarizing the previously reported case studies
Upon discovery of a novel case of dupilumab-treated atopic dermatitis resulting in new onset psoriasis, a call for similar cases was conducted on an online national board-certified dermatologist group. Several physicians reported similar findings and submitted their patient’s cases, with their consent, along with any relevant figures.
Five male patients age ranged from 30-69 were each diagnosed with moderate-to-severe AD. They were initially treated with traditional topical and systemic therapies for atopic dermatitis such as topical steroids, calcineurin inhibitors, and oral immune suppressants. Ultimately, all patients required dupilumab with improvement noted in their first few months of treatment. Psoriaform dermatitis presented at varying timelines. In 4/5 cases, the diagnosis of presumed drug-induced psoriasis was supported with biopsies and pathology consistent with the morphologic changes noted in clinic (Figure 1). In 4/5 cases patients were able to fully recover from psoriaform plaques when dupilumab was discontinued. In some cases, patients were able to restart Dupilumab without reoccurrence of psoriaform findings. However, in one case, the rash persisted despite several treatment attempts (Table 2).
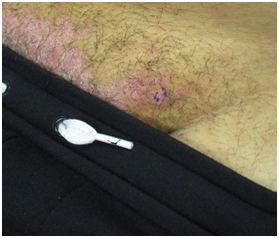
Figure 1 Patient 4 – Psoriasis. Psoriatic Plaques on the mons pubis, that developed after initiating dupiliumab.
|
Patient description |
Time treated AD with dupilumab prior to symptoms |
Biopsy results |
Treatment choice for psoriform lesions when occurred |
|
47 year old male |
6 months |
punch biopsy performed at his initial evaluation demonstrated spongiotic dermatitis |
Dupilumab was discontinued, and he was switched to ixekizumab. Rash worsened on new regiment so was re-started on dupilumab with 300 mg weekly. No lesion reoccurred. |
|
69 year old male |
5 months |
subacute spongiotic dermatitis |
Treated with clobetasol solution and had significant improvement. |
|
32 year old male |
1 year |
psoriasiform dermatitis |
Discontinued Dupilumab. Psoriaform rash persisted. Only minor improvement in rash with phototherapy, excimer laser, and topical steroids. |
|
30 year old male |
6 months |
subacute spongiotic dermatitis |
Discontinued dupilumab and started on secukinumab. Psoriatic plaques resolved after 2 months. At this point, dupulimab was restarted with secukinumab with no lesion reoccurrence. |
|
40 year old male |
9 months |
psoriasis |
Dupilmab continued with methotrexate with minimal improvement. Once discontinued this regiment, switched to apremilast with resolution of psoriasis. |
Table 2 Below is a chart summarizing novel case studies presented above
In this case series, we demonstrate a novel side-effect of dupilumab. The mechanism for drug-induced psoriasiform dermatitis has not been fully elucidated. We support the proposition that this is likely a shift from Th-2 to Th-1 based inflammation induced by dupilumab. It is postulated that by blocking Th-2, dupilumab causes the Th-1 cascade to work in overdrive, eventually leading to Th-1 disease such as psoriasis.1,4,5 Studies have shown that IL-4 is a negative regulator of Th-1 and Th-17 cells, which can inhibit the formation of psoriatic lesions. As a result, there is further support that blocking Th-2 responses with dupilumab through IL-4/IL-13 could result in a shift to Th-1 and Th-17 based inflammatory cytokine cascades that lead to psoriasis.5 It is also postulated that select patients may be more susceptible to this side effect, as they inherently have overactive Th1 pathways.
Close monitoring for this side effect in patients treated with dupilumab should be alerted to dermatologists. There is reassurance in the time-limited nature of the psoriaform side-effect discussed. In the majority of cases, patients are able to fully recover after the offending agent is removed and sometimes patients are even able to restart the medication without further skin lesion development. Further studies are needed to uncover the basis for which patients are more vulnerable to this unexpected result.
The author declares that there is no conflicts of interest.
None.
None.

©2020 Turowski, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.