Journal of
eISSN: 2574-9943


Introduction: Dermatophytic infections are very common superficial fungal infections, which have been reported worldwide. Once considered to be easily treated may become recalcitrant and extensive. The three genera of dermatophytes involved for causing infection are Trichophyton, Epidermophyton and Microsporum. Some of the dermatophytes are cosmopolitan in distribution, while others can be geographically restricted. The epidemiology of dermatophytes may keep on changing due to migration, tourism, war, association with pets and also socioeconomic status.
Material and methods: We conducted a study to find out the causative dermatophytes and clinical types associated with them. The clinical samples in the form of skin scrapings, plucked hair and nail scrapings and subungual debris were collected in the microbiology department of the tertiary care centre. The samples were subjected to 20% potassium hydroxide (KOH) mount and later inoculated on Sabouraud’s Dextrose Agar (SDA) supplemented with chloramphenicol and cycloheximide. The plates were incubated at 25°C and observed for growth every week for four weeks.
Results: We found that tinea corporis was the most common clinical type (61.20%) and was due to Trichophyton interdigitale (T. interdigitale) except one case, which was due to Trichophyton rubrum (T. rubrum). The next in frequency was tinea cruris (24.34%) due to T. interdigitale. Most of the cases of dermatophytosis were extensive without central clearing and with involvement of multiple anatomical sites. There was only one case of tinea capitis (0.65%) due to Trichophyton violaceum (T. violaceum). We did not find any inflammatory lesions or involvement of lymph nodes.
Keywords: Trichophyton interdigitale, Sabouraud’s dextrose agar (SDA), Topical Corticosteroids (TCs), Fixed Drug Combinations (FDCs), Over-the–counter (OTC)
Dermatophytosis is one of the most common infectious diseases worldwide and causes serious chronic morbidity.1 Dermatophytes usually cause superficial infection of the keratinized tissue like skin, hair and nail. The infection is usually superficial in immunocompetent individuals, but the infection can go deeper in immunocompromised individuals either due to AIDS or due to any other cause of immunosuppression. The three-anamorph genera of Trichophyton, Epidermophyton, Microsporum and telomorph genera of Trichophyton species of Arthroderma benhamiae are the causative organisms.
In the newly taxonomic tree, Sybren de Hoog et al. (unpublished data) classifies dermatophytes into six clades based on multilocus genetic analysis, Trichophyton, Epidermophyton, Nannizzia, Microsporum, Lophophyton and Arthroderma.2 Some changes have been made in the nomenclature of Trichophyton mentagrophyte complex. Trichophyton mentagrophytes var interdigitale is now known as Trichophyton interdigitale (anthropophilic strain) and Trichophyton mentarophytes var mentagrophytes is now called Trichophyton interdigitale (zoophilic strain).3
Dermatophytosis is among the most common causes of skin diseases in the world, and the real prevalence is probably underestimated.4 The current scenario of dermatophytosis is formidable. Patients present with multiple, atypical, chronic, recurrent and recalcitrant lesions, and may involve the family members of three generations. Most of the reports from different regions of India,5‒8 have found T. rubrum to be the predominant species associated with dermatophytosis, but in our area, T. interdigitale is the predominant species. Some of the clinical forms, e.g. tinea faciei and tinea genitalis are being reported with increased frequency.8‒12 Corticosteroids misuse, usually topical, is the important contributing factor to this epidemic-like situation.13
Inclusion criterion
All patients suspicious or with clinical diagnosis of dermatophytosis were sent to mycology section of microbiology department of the tertiary care centre. Those patients, who had applied any cream/ointment or oil over the lesions, were requested to come the following day after bath with simple soap and water and without applying anything. The scales are better visualized without applying anything. The site from where the sample was to be collected was scrubbed with 70% alcohol. According to the site and condition of the lesion, the samples were collected, e.g.
The skin scrapings and nail clippings were collected in sterile petri dishes, and when scrapings were few, the sample was directly inoculated on SDA supplemented with chloramphenicol and cycloheximide. The scales were gently planted in SDA. The petri dishes were kept for a month in case repeat culture was needed. Macroscopic and microscopic characteristics were studied. Lacto phenol cotton blue (LPCB) mount was prepared to study microscopic characteristics in details. Slide cultures were set up, where needed. Basic tests like urease and in vitro hair perforation tests were performed to differentiate T. interdigitale from T. rubrum.
A total of 100 patients were consented and enrolled during the period from October 2016 to March 2017, which included 63 males and 37 females. Maximum numbers of patients were found in the age group 16-30 (55), 35 males and 20 females. The youngest patient was one-year old female child with tinea corporis and the eldest was 60 years old female with extensive tinea corporis. The study indicates that dermatophytosis is more common in males (63%) than in females (37%). Multiple anatomic site involvement was seen in 46 patients, 30 males and 16 females. The lesions were extensive and without central clearing. The total number of clinical types was 152, which was higher than the total number of patients. Tinea corporis was the commonest clinical type (61.20%) (Figure 1) observed followed by tinea cruris (24.34%), tinea faciei (7.90%) (Figure 2), tinea genitalis (5.26%) (Figure 3) (Figure 4) tinea manuum and tinea capitis (0.65%) (Figures 5) (Figure 6) (Table 1).
Clinical Type |
0-15 years |
16-30 years |
31-45 years |
46-60 years |
>60 years |
Total number of clinical pattern |
|||||
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
||
T. corporis |
4 |
3 |
35 |
18 |
13 |
7 |
5 |
6 |
1 |
1 |
93 (61.20) |
T. cruris |
1 |
- |
15 |
8 |
5 |
3 |
2 |
3 |
- |
- |
37 (24.34) |
T. capitis |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
01 (0.65) |
T. faciei |
3 |
1 |
4 |
4 |
- |
- |
- |
- |
- |
- |
12 (7.90) |
T. manuum |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
- |
01 (0.65) |
T. genitalis |
- |
- |
4 |
1 |
2 |
1 |
- |
- |
- |
- |
08 (5.26) |
T. pedis |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 (0) |
T. unguium |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 (0) |
T. barbae |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0 (0) |
Total |
9 |
4 |
59 |
31 |
20 |
11 |
7 |
9 |
1 |
1 |
152 |
Table 1 Depicting comprehensive information in relation to sex, age group, clinical types and percentage of clinical pattern
Three males had tinea cruris et corporis along with tinea genitalis (Figure 3) and two females (Figures 7B, 7D) had tinea corporis et cruris along with tinea genitalis (Table 2). Majority of the patients clinically presented with extensive steroid modified tinea (Figure 7A-7D) (Figure 8A, 8B) (Figure 3). One male patient in the age group 31-45 year, presented with iatrogenic Cushing’s syndrome with bilateral osteonecrosis of femoral head (Figures 8A, 8B) (Figure 8).
Clinical Type |
0-15 years |
16-30 years |
31-45 years |
46-60 years |
>60 years |
Total number & % of clinical pattern |
|||||
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
||
T. corporis |
2 |
2 |
14 |
8 |
9 |
4 |
2 |
3 |
1 |
1 |
46 (46%) |
T. cruris |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
T. capitis |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
01 (1%) |
T. faciei |
2 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
02 (2%) |
T. cruris + T. faciei |
- |
- |
- |
2 |
- |
- |
- |
- |
- |
- |
02 (2%) |
T. genitalis |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
01 (1%) |
T. corporis + T. cruris |
1 |
- |
11 |
4 |
3 |
2 |
2 |
2 |
- |
- |
25 (25%) |
T. cruris + T. genitalis |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
01 (1%) |
Extensive T. corporis |
- |
- |
- |
3 |
- |
- |
1 |
- |
- |
- |
04 (4%) |
T. corporis + T. faciei |
1 |
1 |
4 |
1 |
- |
- |
- |
- |
- |
- |
07 (7%) |
T. corporis + T. cruris + T. genitalis |
- |
- |
3 |
1 |
- |
1 |
- |
- |
- |
- |
05 (5%) |
T. cruris + T. corporis |
- |
- |
1 |
- |
1 |
- |
- |
- |
- |
- |
02 (2%) |
T. corporis + T. cruris + T. faciei |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
01 (1%) |
Extensive T. corporis + T. cruris |
- |
- |
- |
- |
- |
- |
- |
1 |
- |
- |
01 (1%) |
T. corporis + T. manuum |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
- |
01 (1%) |
T. corporis + T. genitalis |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
- |
01 (1%) |
Total |
7 |
3 |
35 |
20 |
15 |
7 |
5 |
6 |
1 |
1 |
100 |
Table 2 Combination of various clinical types of dermatophytosis with percentage
Out of a total 152 samples, 138 (90.79%) scrapings were KOH positive and 140 (92.11%) culture positive. All 100 patients were culture positive. Out of 140 culture positive samples, 138 (98.57%) were T. interdigitale (Figure 9), 1 (0.71%) Trichophyton violaceum (T. violaceum) and 1 (0.71%) T. rubrum. Out of 100 patients 98 had single or multiple site infection due to T. interdigitale, one male child (1%) had tinea capitis due to T. violaceum and one male adult (1%) had tinea corporis due to T. rubrum. None of the patients had dual infection due to two different dermatophyte species. At least one anatomic site was positive in patients with the involvement of the multiple sites (Figure 10).
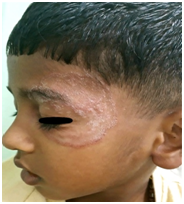
Figure 2 Tinea faciei on the left side of the face of a seven-year-old boy having erythematous double-edged annular scaly plaque without central clearing.
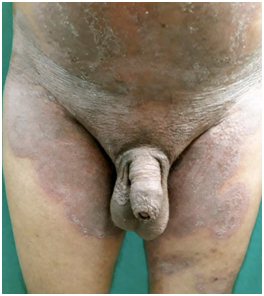
Figure 3 Extensive erythematous, lichenified scaly plaques involving groin, scrotum, prepuce and extending upwards towards abdomen (Tinea cruris et corporis). History of having used topical and systemic steroids and salicylic acid topically.

Figure 4 Tinea cruris with scaly plaque in groin extending to the base of penile shaft and scrotum (tinea genitalis).
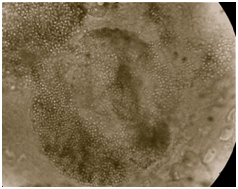
Figure 6 Endothrix pattern of hair invasion due to T. violaceum with appearance of ‘Bag of marbles’ seen in 20% KOH mount. Magnification X400.
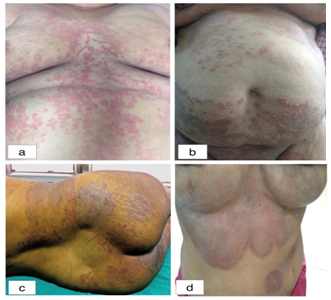
Figure 7 (A) Extensive erythematous, annular and partially annular lesions (B) Extensive tinea corporis with multiple scaly lesions. The lesions over lower abdomen have extended downwards to involve groin (Tinea corporis et cruris) mons pubis, labia majora and labia minora (Tinea genitalis), (C) Healed annular hyper-pigmented plaques with appearance of new lesions at the periphery (Tinea recidivans) (D) Extensive erythematous, hyper-pigmented lesions with hypertrichosis over the front of chest and under breasts, dumb bell shaped double-edged tinea.

Figure 8 Man with extensive dermatophytosis of multiple anatomic sites. Striae over abdomen and thighs. Developed iatrogenic Cushing’s syndrome, bilateral osteonecrosis of femoral head, central obesity and hypertrichosis. History of having used topical and systemic steroids.
According to various studies from the different states of India, T. rubrum is the predominant species.9 Predominance of T. rubrum in India has been shown in central India and all the states below it before 1988.14 Data on the prevalence of dermatophytes before this epidemic-like situation in this area is missing. For the past 6-7 years, this terrible scenario has been continuing. One- third of the dermatology clinic attendance is due to patients with dermatophytosis.
Medical fraternity in India has been observing an increase in the prevalence of dermatophytosis and that two of the difficult to treat recalcitrant, recurrent and chronic dermatophytosis, over the last 3-4 years.15 Here, in our study, the epidemic is due to T. interdigitale (98%). We had one case of tinea capitis due to T. violaceum in the present study. During past four years, we had one case of tinea faciei due to T. violaceum. We have not reported any case of extensive tinea due to T. violaceum though epidemics/outbreaks have been reported to this dermatophyte species.16‒17 Also, we did not find any case of tinea pedis or tinea unguim during this study period. Tinea pedis is relatively uncommon in India and is the least prevalent of all the dermatomycoses.18 Recently, there have been some reports of extensive, atypical chronic and recalcitrant dermatophytosis from West Bengal,7 Gujarat and Tamil Nadu,8 Sikkim,19 Udisha,20 Chandigarh,21 and Rajasthan.22
Besides getting patients with extensive, recurrent and recalcitrant cases of dermatophytic infection with involvement of multiple anatomic sites, we also get patients with local side effects of having used topical corticosteroid, e.g. no central clearing, skin atrophy, striae (Figure 8A), hypertrichosis (Figure 8B), telangiectasia, double edged tinea (Figure 2), tinea pseudoimbricata, tinea recidivans (Figure 7C) and many atypical presentation. We are also getting patients with systemic side effects. One of the patients developed Cushing’s syndrome with bilateral osteonecrosis of femoral head. In some cases, multiple members of the three generations of the family were having dermatophytic infection.
Recently six patients came with the serious side effects of topical and systemic side effects, after being treated by unqualified practioners in the village. They were not enrolled in the study. Two female patients died in intensive care unit due to chest infection. Others were being treated.
Lack of qualified dermatologists, more so in rural area, is also compounding the problem of irrational use of corticosteroids.23 Also, it has been sometimes observed that even trained physicians and dermatologists are prescribing either the wrong strength of TCs or for wrong indication.23 To fight the challenges of current dermatophytosis, a theme related conference was organized at Post Graduate Institute of Medical Education and Research (Chandigarh) in 2017.24 Mixing of topical steroid in fairness creams has lead to tinea incognito and topical steroid damaged/dependent face (TSDF).25 Dermatologists are making all possible efforts to create awareness among the public and to ban all beauty products and creams having topical steroids as one of the ingredients.
Factors contributing to the epidemic-like scenario in India
How to curb the epidemic
The country’s Drug Technical Advisory Board banned 328 FDC drugs on September 13, 2018, including skin care cream PANDERM (Figure 10) containing clobetasol propionate, terbinafine, ofloxacin and ornidazole, as there is no therapeutic justification for the ingredient of this drug and banned it in public interest.
The current scenario of this steroid modified tinea is quite critical. Unless a holistic approach to limit this epidemic is implemented, it will lead to more intimidating consequences and when it will cease, difficult to predict. Adequate epidemiological investigations including molecular characterization to reveal the genetic relatedness and susceptibility of the suspected epidemic strains should be performed. Strict regulation against the availability of FDCs available as OTC. Funding from the government for the poor patients, though it will cause lot of financial burden, but seems mandatory.
Authors thank all the individuals who contributed to this study. No funding was received for the study.
Authors declare that there is no conflict of interest.
The study was conducted in accordance with research ethics.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.