Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 3
1International Hellenic University (IHU), Department of Environmental Engineering, Greece
2PO Box 48 K, 57500 Epanomi, Greece
3Fisheries Research Institute (FRI), Greece
Correspondence: Sofia Galinou-Mitsoudi, International Hellenic University (IHU), Department of Environmental Engineering, PO Box 141, 57400 Thessaloniki, Greece
Received: September 19, 2023 | Published: September 29, 2023
Citation: Galinou-Mitsoudi S, Samara E, Manousis T, et al. The European wing oyster Pteria hirundo (Linnaeus, 1758) (Mollusca: Pteriidae): a known species with an unknown life mode. J Aquac Mar Biol. 2023;12(3):234-238. DOI: 10.15406/jamb.2023.12.00378
The European wing oyster Pteria hirundo is studied in terms of autecology: habitat, age and growth, substrate and associated fauna, relationships, and distribution and abundance in the North Aegean Sea of Greece by means of extensive collection, photography, biometry, and application of von Bertalanffy growth equation (VBGE), logistic 3P Prediction Model, and linear regression analysis on morphometric parameters. Results, such as the variety of sessile corals, hydrozoa, bivalves and sea urchins that the species uses as substrate, and its epibiotic polychaetes and bryozoa, are presented and discussed.
Keywords: distribution, autecology, morphometry, age, associated fauna, Aegean Sea, Greece
Pteria hirundo (Linnaeus, 1758), the European wing oyster, is a well-known bivalve of the sublittoral zone from 20-150 m1 or up to 300 m2 with numerous references supplying information on the species habitat and distribution. While the species is cultured in Brazil,3 its exploitation in Greece is very limited.4 Information on the life history of P. hirundo wild populations from the Mediterranean is lacking, presumably due to difficulties in its collection as the species is rather uncommon5 and lives in habitats of mainly hard substrates of some depth as epizootic on soft and hard corals. This study adds to the knowledge of European wing oyster’s autoecology, habitat, age, growth and distribution in the Aegean Sea.
Individuals of Pteria hirundo were sporadically collected (Figure 1) during the period 2006-2021 among by catches of commercial fishing gears of: i) trammel nets and ii) trawls, in the North and Central Aegean Sea.
Recorded information referred to depth, substrate type, and surface of collection area depending on the trawl and trammel nets.
The dimensions of undamaged specimens were measured (length, height, width) with an accuracy of 0.05 mm, and the individuals were weighted (total, body, valves) (as dry weight after 48 h, in 80°C oven) (accuracy 0.001 g).
Relationships between dimensions, dimensions and weights were described with linear regressions of the general form
For the relationships between dimensions, all specimens of this study were used, while for the relationships between dimensions and weight the specimens of the most numerous collection were used (trammel nets, September 2006).
The age was estimated by counting the winter dense zones6 both on valves and valve ears as well as by using back calculation when the shells’ condition permitted. For the study of the age, specimens were collected from the N. Aegean Sea during April to September when the last winter dense zone on the valves is completed and the new one will be appear later. The theoretical growth curve (von Bertalanffy equation and its parameters L∞, k and to) was calculated according to.7–9 Organisms as substrate, associated fauna and epibionts of Pteria hirundo were identified at the genus level, at least.
Ecological aspects
The specimens of Pteria hirundo were collected from depths of 25-443 m with the majority of individuals from 90-324 m of the North and Central Aegean Sea (Figure 1 and Table 1). The species abundance was higher on substrates with Antipathes corals and lower on Eunicella singularis ones (Table 1). The trammel nets were 4000 m long, while in September 2006 all individuals (36) were collected on a 10 m long part of the net attached with their strong byssus (Figure 2d) on Antipathes arms (Figures 2a and e). The mean length of the trawling swept/hauling distances was 3858±sd1570 m from where 0-11 P. hirundo individuals were collected (Table 1).
|
Time |
Coordinations (the middle of nets/hauling) |
Area (symbols in the map of Fig. 1) |
Fishing gear |
Mean Depth (m) |
No of Indivi-duals |
|
September2006 |
39o 55’ 800 23o 58’ 350 |
NW Aegean Sea (N2) |
trammel nets |
90-95 |
36 |
|
April-May 2007 |
40o 27’ 320 22o 45’ 031 |
NW Aegean Sea (N3) |
trammel nets |
25 |
1 |
|
April-May 2007 |
40o 13’ 000 23o 26’ 000 |
NW Aegean Sea (N1) |
trammel nets |
70 |
5 |
|
July2019
|
40o 36’ 979 25o 07’ 825 |
Thracian Sea (7) |
trawl |
132 |
2 |
|
39o 46’ 564 25o 08’ 422 |
C Aegean Sea (14) |
97 |
7 |
||
|
39o 05’ 100 25o 15’ 331 |
C Aegean Sea (19) |
324 |
11 |
||
|
August 2020 |
38o 28’ 331 25o 16’ 473 |
C Aegean Sea (25) |
523 |
0 |
|
|
39o 01’ 814 23o 45’ 752 |
C Aegean Sea (35) |
360 |
1 |
||
|
39o 00’ 934 23o 11’ 747 |
C Aegean Sea (37) |
45 |
2 |
||
|
38o 49’ 163 23o 06 466 |
C Aegean Sea (39) |
443 |
1 |
||
|
40o 10’ 320 23o 16’ 089 |
NW Aegean Sea (42) |
66 |
3 |
||
|
39o 49’ 413 23o 12’ 653 |
NW Aegean Sea (50) |
108 |
3 |
||
|
39o 50’ 670 23o 31’ 979 |
NW Aegean Sea (52) |
364 |
1 |
||
|
June 2021 |
40o 38’ 965 25o 23’ 864 |
Thracian Sea (6) |
84 |
0 |
|
|
39 o 42’ 965 25o 01’ 180 |
C Aegean Sea (15) |
187 |
3 |
||
|
40o 31’ 273 24o 23’ 503 |
Thracian Sea (64) |
311 |
0 |
Table 1 Collection details for Pteria hirundo of this study. C =Central
Pteria hirundo was found to be strongly attached by the byssus on a variety of sessile invertebrate organisms (Figure 2 and 3) such as the corals Antipathes dichotoma Pallas, 1766 (Figure 2a and e) and Eunicella singularis (Esper, 1791) (Figure 2b), the Hydrozoa Aglaophenia cf. elongata (Fig. 3a), Lafoea cf. dumosa (Fig. 3b) and Nemertesia cf. antennina (Figure 3c), the bivalve Neopycnodonte cochlear (Poli, 1795) (Figure 3d) and on sea urchins (Figure 3e). P. hirundo individuals were also found settled on branches of submerged trees (Figure 2c) and on fishing lines. P. hirundo itself became substrate for other benthic organisms (epibionts) such as Anomiidae, Polychaeta and six Bryozoa species (Figure 3f-k) three of which, namely Bugulina flabellata (Thompson in Gray, 1848), Fenestrulina orientalis Liu & Liu, 2001 and Schizoporella cf. japonica Ortmann, 1890, are referred for the first time from Greece based on Gerovasileiou & Rosso10. As associated molluscan fauna with P. hirundo were found to be the bivalve Anadara gibbosa (Reeve, 1844) (Figure 4a) and the gastropods Pseudosimnia carnea (Poiret, 1789) live (Figure 4b), Ranella olearium (Linnaeus, 1758) live (Figure 4c), and Odostomia aff. schrami11 (Figure 4d). The later O. aff. schrami was collected also live from the surface of a live deep water sea urchin associated with P. hirundo clusters.
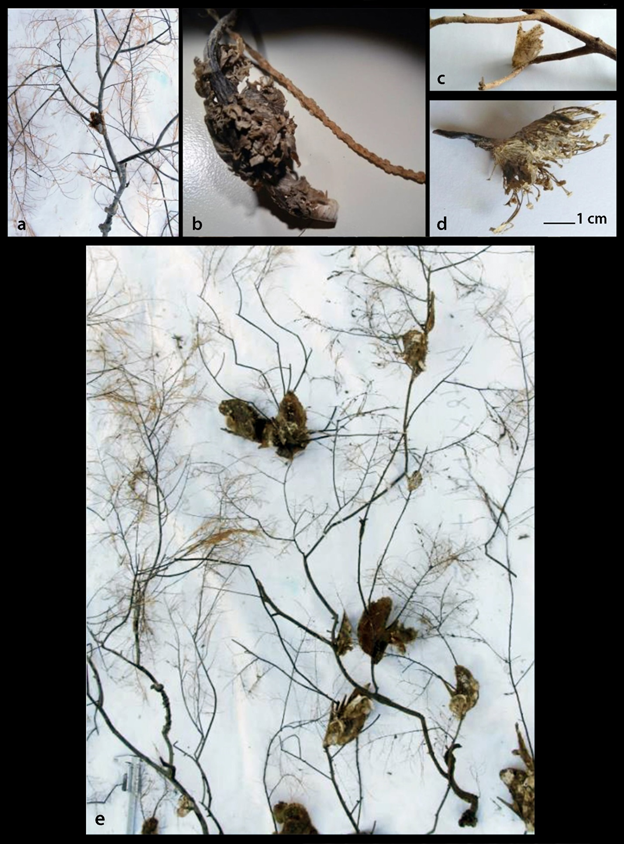
Figure 2 Pteria hirundo substrates: a. The coral Antipathes dichotoma with an attached P. hirundo individual; b. The coral Eunicella singularis holdfast (“root”) with a part of its dry branch; c. Branch of a land tree; d. Byssus; e. P. hirundo individuals attached on Antipathes dichotoma branches when removed by the trammel nets in September 2006.

Figure 3 Substrates of Pteria hirundo. a-c. Hydrozoa: a. Aglaophenia cf. elongata Meneghini, 1845; b. Lafoea cf. dumosa (Fleming, 1820); c. Nemertesia cf. antennina (Linnaeus, 1758); Bivalvia: d. Neopycnodonte cochlear (Poli, 1795) and Antipathes sp. coral; Echinodermata: e. Cidaris aff. cidaris (Linnaeus, 1758); Bryozoa epibionts: f. Bugulina flabellata (Thompson in Gray, 1848); g. Bugula neritina (Linnaeus, 1758); h. Cradoscrupocellaria cf. bertholletii; i. Fenestrulina orientalis Liu & Liu, 2001; j. Schizoporella errata (Waters, 1878) ; k. Schizoporella cf. japonica Ortmann, 1890.
The larval shell shape of Pteria hirundo is shown in Figure 4e. The size of the prodissoconch I and II is 150 μm and 420 μm, respectively. On the bases of the late ontogenetic stages the shell is easily recognized as inequivalve. The valves of P. hirundo differ in the density and the size of the aragonite prisms. The right (larger) valve consists of significantly more and smaller prisms than the left one (Figure 4f and g).
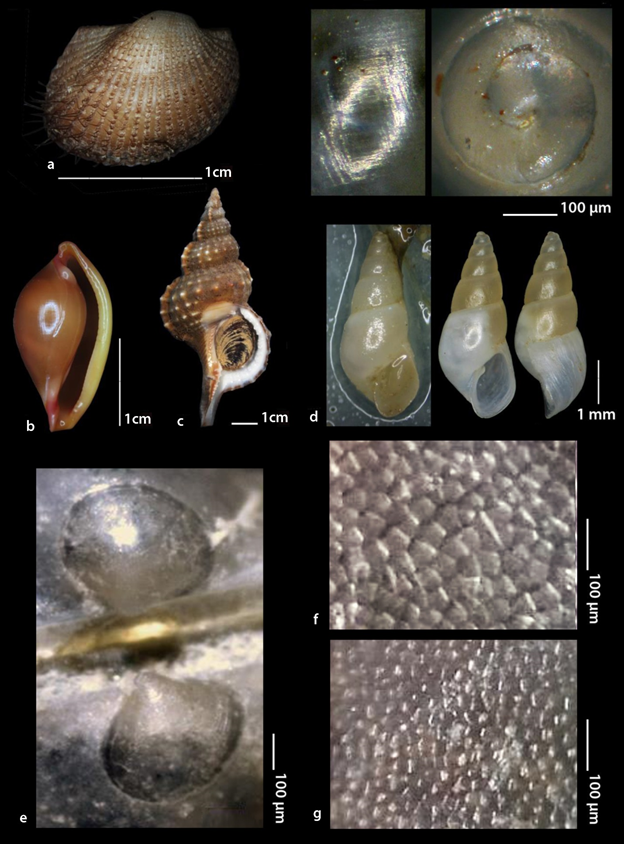
Figure 4 a-d. Pteria hirundo associated molluscs in this effort: a. Anadara gibbosa (Reeve, 1844) live; b. Pseudosimnia carnea (Poiret, 1789) live; c. Ranella olearium (Linnaeus, 1758) live; d. Odostomia aff. schrami van Aartsen, Gittenberger E. &Goud, 1998 live; e. The larval shell of P. hirundo (length 420 μm) at the umbo area of an adult individual, the upper valve is the left one; f. Prisms density of P. hirundo shell close to ventral margins of the left valve; g. Prisms density of P. hirundo shell close to ventral margins of the right (larger) valve.
From the most numerous collection (trammel nets, September 2006), the minimum and maximum length of the specimens was 0.38 cm and 10.62 cm, respectively. The valves were thin and fragile with damages at the margins due to the fishing gears. Therefore, the most accurate shell dimension was that of the width that gives the smaller standard deviation (Table 2). The shell dry weight represents 92.7% of the total dry weight and the body dry weight only the 7.3%. The descriptive statistics of all measurements is presented in Table 2.
|
Variable |
N |
Mean |
StDev |
Minimum |
Maximum |
|
Length (cm) |
29 |
6.232 |
2.794 |
0.380 |
10.620 |
|
Height (cm) |
33 |
4.517 |
1.736 |
0.300 |
6.620 |
|
Width (cm) |
35 |
1.323 |
0.481 |
0.300 |
1.880 |
|
TDw (g) |
36 |
9.060 |
6.460 |
0.000 |
21.75 |
|
BDw (g) |
35 |
0.681 |
0.449 |
0.002 |
1.651 |
|
ShDw (g) |
35 |
8.640 |
5.950 |
0.090 |
20.800 |
Table 2 Descriptive statistics of Pteria hirundo biometry. TDw: Total Dry weight; BDw: Body Dry weight; ShDw: Shell (both valves) Dry weight for N individuals
Independently of the fishing gear and the recorded period, the size of the individuals in terms of width is presented in figure 5, and shows larger individuals in the NW Aegean Sea.
The dimensions (length, height, width) of the collected specimens by trawls during the period 2019-2021 did not differ statistically (ANOVA one way: P0.05 = 0.424 for length, 0.269 for height, 0.629 for width).
The relationship of width with the other two valve dimensions (length and height) shows strong correlation with an R2>93%, and the relationships (R2>93%) of width vs. dry weights of P. hirundo are presented in Table 3.
|
N |
Linear regression logy= loga + blogx |
R2 (%) |
ANOVA0.050 probability (P) |
Standard error of b (95% C.I. of b) |
Allometry |
|
56 |
logL = 0.7114 + 1.042 logW |
93.34 |
0.000 |
0.0375 (0.967 – 1.117) |
0 |
|
29 |
logL = 0.081 + 1.080 logH |
97.87 |
0.000 |
0.0193 (0.998 – 1.075) |
0 |
|
33 |
logTDw = 0,5285 + 2,7960 logW |
97.38 |
0.000 |
0.0810 (2.631- 2.961) |
- |
|
33 |
logBDw = -0,6233 + 2,801 logW |
93.81 |
0.000 |
0.1270 (2.542-3.060) |
0 |
|
34 |
logShDw =0,4900 + 2,7482 logW |
96.39 |
0.000 |
0.0926 (2.559-2.937) |
- |
|
33 |
logShDw = -0,0295 + 0,9960 logTDw |
99.98 |
0.000 |
0.0025 (0.991-1.001) |
0 |
Table 3 Linear regressions of morphometric parameters (L = length, H = height, W = width) of body (B), shell (Sh) and total dry weight (TDw), R2: determination coefficient (%), C.I.: confidence intervals of b for 95% probability. Allometry (-/0): negative/isometric
The estimated age of 36 Pteria hirundo individuals was 12+ years at a length of 7.57 cm. The parameters of theoretical growth curve, von Bertalanffy equation, were calculated as follows:
The Fort-Walfort regression equation was (R2 = 94.88%, P0.05 =0.00) and consequently the parameters k, and to were calculated: k = -lnb=0.156/year, = a/1-b = 1.79 cm that corresponds to an infinitive length = 9.44 cm, to = -a/b constants of regression age vs. –ln(1-Lt/ )9 is found to be -0.701. Substituting the values of these parameters, the von Bertalanffy equation is: (Figure 6).
The winged pearl oyster Pteria hirundo seems to be widely distributed in the Aegean Sea at a depth range of 25-523 m. The specimens from the open and deeper waters of North (Thracian) and Central Aegean Sea were smaller in comparison with the individuals from the gulfs of NW Aegean Sea. The optimal conditions for successful survival and growth of post larvae to juvenile ages of the species in a farm were found to be a combination of sea water temperature 23 °C and salinity 35 ‰.3 The period after reproduction of natural populations in the Aegean Sea has to be the summer, based on the occurrence of very small specimens (~5 mm) in August and September.10,11 The summer temperature and salinity of the North and Central Aegean Sea are 20-24 oC and 32-38‰, respectively; while in the NW Aegean are 24 oC and 35-36 ‰, respectively.12 The favorable environment for the early age stages of P. hirundo is rather that of NW Aegean where the species was found to be more in abundance. The maximum length of the valves, 11 cm, was referred by Pusateri in 2003, from the Mediterranean Sea,13 which is very close to the largest specimen of this study (10.62 cm) and close to the theoretical L∞ (9.44 cm). The dissimilarity between the size and the number of aragonite prisms between the two valves of the congeneric species Pteria penguin (Röding, 1798) was described in detail by Harper & Checa14 who attribute the difference to the observed higher organic content of the right valve prismatic layer having as a result an increased number of smaller prisms. The calculated life span of P. hirundo is more than 15 years, with a low growth rate (k = 0.156/year), quite similar to other congeneric species e.g. P. penguin (k = 0.090-0.380/year)15. The species total weight rises after a length of 2 cm at an age of about 2 years (Figure 7) and rather indicates the age of fist reproduction at a length of >6 cm, as the inflection point of the prediction model Logistic 3P is 6,42 cm (Figure 5). The species of Pteria seems to be epizootic, as the majority of the specimens of this study (see Figures 2 and 3) were attached to stems of gorgonians. It has been observed that the most abundant species of the Bivalvia group collected from a depth of 50-100 m with a trawl in the Adriatic Sea was P. hirundo (2922.19 individuals/km2 and weighted 35.70 kg/km2)16 while the corresponding abundance of P. hirundo in the Aegean Sea was only 0-11 individuals per effort, perhaps due to deeper waters and less favorable conditions in general.
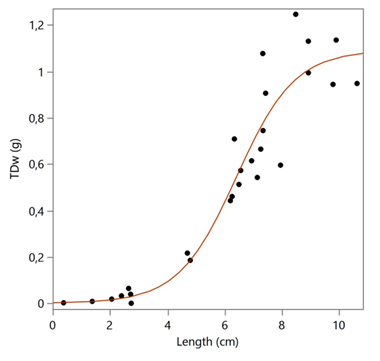
Figure 7 Logistic 3P Prediction model for the total dry weight (TDw) changes with the size (Length).
Odostomia schrami is an E Atlantic inhabitant initially reported as a worn out shell (spec, nov., holotype NNM 57490) from off Mauritania11. Since then, a live specimen was collected from NE Thermaikos Gulf, N Aegean Sea17 and now a much larger specimen from a different location (station 7) of the N Aegean Sea. As the later finding represents a rather adult individual and these is no relevant image documented report available in the literature we added the “aff.” to our specimen. If it is indeed O. schrami, these two findings from two distant locations are indicative of an established population, and gave us the opportunity to examine in detail its characters, among them, the size of the protoconch (embryonic shell 245 μm, larval shell 315 μm, maximum diameter), the finely spirally striated surface of the shell, the flared lower part of the outer lip, the animal colour and its habitat in the E Mediterranean Sea. Concerning the collected sea urchins, we avoid identifying them as one of the two deep sea Mediterranean species Cidaris cidaris (Linnaeus, 1758) or Stylocidaris affinis (Philippi, 1845) because their spines were broken due to trawling. The relative length of those spines to the diameter of the dermal skeleton comprises a useful tool in the discrimination and specific identification of those two similar species18. The use of cf. for some species of this work (e.g. hydrozoa and bryozoa) is due to the worn samples due to trawling.
Further investigations are needed to complete the life cycle of the species and its survival under the present climate and anthropogenic pressures.
The authors are grateful to the unknown reviewers as well as to the fishermen Manolis and Theodosia who provided the Pteria specimens from trammel nets, and to Prof. Dimitris Petridis for the Logistic 3P Prediction Model.
The authors declare that there are no conflicts of interest.

©2023 Galinou-Mitsoudi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.