Journal of
eISSN: 2378-3184


Research Article Volume 2 Issue 3
Independent Aquaculture Consultant, USA/Spain
Correspondence: Gonzalo Ill, Tel +34-610425220
Received: January 07, 2015 | Published: July 1, 2015
Citation: Illán G (2015) Risk Factors Associated with the Presence of Parasitic Diseases in Cultured Tench (Tinca tinca L.) from the Tormes River (NW Spain). J Aquac Mar Biol 2(3): 00027 DOI: 10.15406/jamb.2015.02.00027
The present study aims to assess the epidemiology of fish parasites in cultured-fish populations. This study was carried out during a three year period (2005-2007) at a tench (Tinca tinca L.) fish farm located in the Northwestern region of Spain. 114 fish as well as 14 physicochemical and microbiological water parameters were analyzed in order to determine the main impact of both the host and the environmental parameters on the presence and the abundance of parasites in their host (host-parasite relation).Four different parasites, namely Ichthyophthirius multifiliis, Tripartiella sp., Myxobolus sp. and Gyrodactylus sp., were isolated, being the ciliates, with up to 100% of prevalence, the most abundant, with only 5.56% and 2.63% of the fish infected by the myxozoan and the monogenean, respectively. With the exception of the high alkalinity registered at the fish farm (pH > 9.0), the rest of the values fell within the recommended water quality parameters for cyprinids. The statistical and epidemiological analysis revealed that size, somatic condition and sex (intrinsic parameters) in addition to several environmental factors, linked to seasonality and poor water quality, have significant effect on the host-parasite relationship, increasing the likelihood of the parasite presence (p<0.050). We conclude that, in temperate climates, the development, reproduction and transmission of the parasites are triggered under the most stable conditions of the ecosystem (hydrological, physicochemical and biological) i.e. the warmer periods of the year, when the cyprinid populations display a more active and gregarious behavior. The results of the present study will also help understanding both the epidemiology and the parasitology of fresh water cultured-fish, and, what is more important, will help designing management strategies and procedures for preventing parasitic diseases.
Keywords: Epidemiology, Parasitology, Risk factors, Aquaculture, Fish, Tench, Tinca, Parasites
CFU, Colony Forming Unit; DO, Dissolved Oxygen; FTU, Formalized Turbidity Unit; K, Condition Factor; n, Sample Size; OR, Odds Ratio; pLR, P-Value According To Likelihood Ratio; pX2, P-Value According To Chi-Square (χ2) Test; pKW, P-Value According To Kruskal-Wallis Test; pMW, P-Value According To Mann-Whitney Test; ppm, Parts Per Million; µS, Micro Siemens
Tench (Tinca tinca L.) is a fresh and brackish-water fish species of family Cyprinid of Eurasian distribution. In the Iberian Peninsula, it is widely distributed throughout all the river basins, mainly in shallow and lentic aquatic environments, though its populations are rather scarce.1 It can also tolerate low dissolved oxygen levels2 and during the winter, when water temperature drops down significantly, this gregarious omnivorous fish may bury half way into the pond bottom mud and stay in a dormant sedentary state until spring, the spawning season.3 This fish is mainly cultivated in the northwestern areas of Spain for its good quality meat as well as for being a valuable recreational ornamental species. Its culture is intensifying across Europe, with translocation of fish and their diseases as a result.4 Fish can act as definite or intermediate host for parasites. Though most of them behave as saprophytes, these parasites, under certain conditions, may become pathogenic and pose a serious threat to its health status. Thus, any slight, natural or anthropogenic, changes in the rearing conditions can modify the host-parasite relationship leading to outbreaks and high mortalities.5
The increasing impact of some parasitoses on fish health has enhanced the need for deeper studies on piscine parasite fauna especially when knowledge on fish parasites and their impact on fish are currently very scarce. These deeper studies on the parasite-host-environment system will allow not only a more efficient treatment, but also prevention. This three-way interaction, known as disease epidemiological chain,6 can be observed and analyzed using the valuable tools of the epidemiological investigation7 to provide useful information on the nature and extent at which certain factors may influence the process of infection. In farmed hosts, like the ones of the present study, culture conditions and management practices are the determinants of epidemiological factors. The bio-geographical location, characterized by abrupt seasonally changes in environmental factors, mainly water temperature, may also play an important role. The present cross-sectional epidemiological study was designed in a tench fish farm located in the Northwestern region of Spain in order to provide the fish farmers with more information on how to deal with some parasitoses (parasitic diseases) that affect their cultured fish. The general objective of such a study was to determine the impact of both the microhabitat (host) and the microhabitat (environment) parameters on the presence of the different taxonomic groups that we found. More specifically, we aimed to establish the possible associations of the factors involved and the infection prevalence/intensity of the parasites, as well as the quantification of the risk or likelihood associated with their presence.
Area of study
Samples were collected from a tench (Tinca tinca L.) fish farm facility atthe Duero Basin, Tormes River (Figure 1), that is located 13 km away from Salamanca (municipality of Machacón, Northwestern Spain), at 810 m of altitude. The main source of water supply to it is an artesian well then the channel of Villagonzalo. The ponds are made of cement, with the bottom partially covered with soil bottom and excessive aquatic vegetation, being microalgae and invertebrates the main source of nourishment for the omnivorous fish.
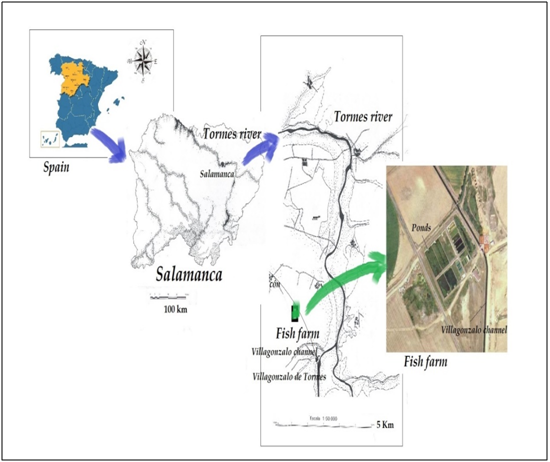
Figure 1 Map showing the geographical situation of the sampling point investigated in Duero river basin, northwestern Spain
Samples collection and analysis
114 individuals of tench fish were captured along a three-year period (2005-2007), on a seasonal basis, for the cross-sectional epidemiological survey on fish parasite fauna. The winter was always skipped due to the fish’s inactive behavior. Water samples were also collected for the determination of physicochemical and microbiological water parameters.
Sampling Fish sampling: Since it was not feasible to gather information straight from the whole piscine population, a representative sample from the target population was randomly chosen, establishing the individual fish as the sample unit. The randomized sample was then stratified according to the studied factors. The sample size was calculated using the epidemiology software “WinEpi”8 and statistically corresponds to a 100% probability of detection of an infected fish, considering a minimum theoretical prevalence of 20% with 95% confidence level. Fish were caught using fishing-traps then transported to the lab alive in clean plastic bags (with 1/3 of its volume filled with water) inside portable refrigerated coolers. Once at the laboratory, fish were weighed, measured and sacrificed by chilling on ice and spinal cord severance or by exposure to anesthetic MS-222 (Sigma®, St Louis MO, USA).Then they were necropsied and examined in less than 24 hours as recommended by other authors.9-11 When it was not possible to work with fresh samples, the organs/tissues were immersed in fixative solutions, usually 10% neutral buffered formalin. External surfaces of the fish were examined first by scraping the integument with a scalpel, especially in case of visible wounds or cysts then the extracted tissues and mucus were deposited onto glass slides. Before placing the glass cover on, a drop of mucolytic 10% potassium hydroxide solution was added. For examining the internal organs, the body cavity was laid open using scissors and scalpel. The digestive tract was open lengthwise (adding 0.8% physiological saline afterwards), then small pieces of the target organs like the liver and the kidney were squashed between glass and cover slides.
Water sampling, in situ measures and analytical methodology: Water quality was monitored seasonally (five times) along the study. This was done by collecting 500 mL water sample (seasonally) from the intermediate zone of the pond using sterile borosilicate glass bottles. The sampling bottles were then transported to the laboratory under cooling conditions where both the physicochemical and the microbiological analyses were performed within a 24 hours period. In order to avoid subsequent changes in time, some parameters were measured in situ using different electronic devices equipped with the right probe: conductivity (µS.cm-1) using a conductivity meter, turbidity (formalized turbidity unit, FTU) with a portable turbidity meter and dissolved oxygen (mg/L) and water temperature (ᵒC) with a portable oxi-meter. Back at the laboratory, alkalinity (kit Visocolor AL7, Macherey-Nagel®) and hardness (kit Aquamerck 1.08011.001 Merck®) were determined by the volumetric determination of their concentrations (mg/L). Different commercial kits (Visocolor ECO) and a portable photometer (Filter Photometer PF-11, Macherey-Nagel®) were also used to determine the concentrations (mg/L) of ammonium (Ammonium Test, 0.2-3 mg/L NH4+), nitrites (Nitrit Test, 0.02-0.5 mg/L NO2–), nitrates (Nitrat Test 4-120 mg/L NO3–) and total phosphate (Phosphat Test, 0.2-5 mg/L PO4-P).
To determine the microbiological quality of the water, different work protocols were followed.12,13 The quantitative determination of faecal indicator organisms was made by film filtration and subsequent seeding on different culture media at an optimum temperature for each of them. This method counts on passing a volume of 100 mL of sampled water through a cellulose acetate filter membrane (0.45µmmesh size), hold onto a sterile filter holder place, with the help of a microfiltration pump (Millipore®). The selective culture media and incubation temperatures were as follows: mEndo Agar Medium (Difco®) for the count of total coliforms, at 37 ± 1ᵒC for 24 ± 2h; mFC Agar Medium (Cultimed®) for the count of faecal coliforms, at 44 ± 0.5ᵒC for 24 ± 2h; and Slanetz & Barley Medium (Cultimed®) for the count of faecal streptococci, at 37 ± 1ᵒC for 48 ± 3h. The final results were expressed in colony forming units (CFU) present in 100 mL of water. For the determination of the total aerobic (mesophilic) micro-organisms 0.5 and 1.0 mL of sampled water were deposited in duplicate on sterile Petri dishes and then deep seeded with 20 mL of nutritive Plate Count Agar Media (Cultimed®); then the samples were incubated at 22 ± 2ᵒC for 72 ± 3h and at 37 ± 2ᵒC for 48 ± 3h, respectively. Also, 20 mL of each water sample was placed into a borosilicate glass tube (Pyrex®), then thermally treated (80ᵒC for 15 min, cooling to 45ᵒC afterwards), filtered and cultivated on Sulphite-Polymixine-Sulfacidine(SPS) Agar (Cultimed®) under anaerobic conditions at 37 ± 1ᵒC for 48h.
Parasitological methods: Here are some of the techniques and standard procedures routinely used in the diagnosis of fish parasite diseases. First, the presence of ectoparasites was investigated by skin and fins’ scraping using a surgical blade and a glass slide; then, using a pipette, a physiological saline (0.8% NaCl) or a potassium hydroxide solution (10% KOH) was introduced between the sample and the glass slide by capillarity, and the preparation was observed under the light microscope, working with the trim of the capacitor almost closed. Fish organs and big parasites (> 1mm) were observed using a stereomicroscope Zeiss® mode Stemi DV4, equipped with continuing magnifying lenses (from 8X up to 32X) and ocular lenses (10X/20X). For a more detailed observation of fish organs and tissues and smaller parasites (< 1mm), an optic microscope Olympus® CX31, equipped with four objective lenses (4X, 10X, 40X and 100X) was used. Some of the isolated parasites were also identified using the scanning electron microscope (SEM) JEOL Ltd. Model JSM-6480 lv, with a 0.3 nm optical resolution, at the University of Leon Microscopy Service.
Fish were considered infected when at least one parasite was detected on any mount or preparation. The terms infection prevalence and intensity were used as in other authors.14-16 The percentage of infected fish in a given sample was referred to as sample prevalence and was calculated using the statistical software SPSS 15.0 for Windows (Chicago, USA), which was then extrapolated to population prevalence (95% confidence intervals) using the epidemiology software WinEpi,8 taking into account the corresponding sample sizes (n). We refer to intensity as the number of parasites of a particular species who live on/inside a single infected host. Our criteria to evaluate the intensity were based on other authors,17,18 who considered the general effect that one or more different parasites could cause on the host. According to this, a quantitative variable was transformed into a semi-quantitative one, following the rule:
Epidemiological survey and data base design: The epidemiological survey was based on two questionnaires that were designed with the aim of gathering the information to be further analyzed. Such information was then entered in Excel spreadsheets, and includes:
Data on the fish (Appendix 2).
|
Variable |
Type of variable – value |
|
General characteristics |
|
|
Sampling point (municipality) |
Nominal |
|
Location |
Nominal |
|
Sampling date |
Date |
|
Season |
Ordinal |
|
Physicochemical parameters of the water |
|
|
Temperature (ᵒC) |
Quantitative |
|
Dissolved Oxygen (ppm) |
Quantitative |
|
pH |
Quantitative |
|
Conductivity (µSiemens/cm) |
Quantitative |
|
Turbidity (FTU)* |
Quantitative |
|
Alkalinity (mg/L HCO3-) |
Quantitative |
|
Hardness (mg/L Calcium and Magnesium CO32- and HCO3-) |
Quantitative |
|
Ammonium (mg/L NH4+) |
Quantitative |
|
Nitrites (mg/L NO2-) |
Quantitative |
|
Nitrates (mg/L NO3-) |
Quantitative |
|
Total phosphates (mg/L PO4-P) |
Quantitative |
|
Microbiological parameters of the water |
|
|
Total Coliforms (CFU/100 mL) |
Quantitative |
|
Faecal Coliforms (CFU/100 mL) |
Quantitative |
|
Faecal Streptococci (CFU/100 mL) |
Quantitative |
|
Sulphite-reducing Clostridia (CFU/20 mL) |
Quantitative |
|
Total Aerobes at 37ºC (CFU/mL) |
Quantitative |
|
Total Aerobes at 22ºC (CFU/mL) |
Quantitative |
Appendix 1 Questionnaire 1: Data on the characteristics of the sampling point and the water quality.
Ppm: Parts Per Million; FTU: Formalized Turbidity Unit; HCO3-: Bicarbonates; CO32-: Carbonates; CUF: Colony Forming Units
|
Variable |
Type of variable – value |
|
|
|
Characteristics of the fish |
|
|
|
|
Sex |
Dichotomous |
|
|
|
Total length (cm)1 |
Continual |
|
|
|
Weight (g) |
Continual |
|
|
|
Condition factor (K)2 |
Continual |
|
|
|
Reproductive status (spawning) |
Dichotomous |
|
|
|
Parasites |
Infection |
Place (body) |
Intensity |
|
Ichthyophthirius multifiliis |
Dichotomous |
Nominal |
Ordinal |
|
Tripartiella sp. |
Dichotomous |
Nominal |
Ordinal |
|
Gyrodactylus sp. |
Dichotomous |
Nominal |
Ordinal |
|
Myxobolus sp. (internal organs) |
Dichotomous |
Nominal |
Ordinal |
Appendix 2 Questionnaire 2: Data on the fish and the parasites
1Total length: length of the fish from the top of the head to the tip of the tail fin.
2Condition factor (somatic condition or Fulton index), according to Fulton equation: K = 100 x weight/lenght3
Data analysis
Once the database was created and filled in with all the required information, a global spreadsheet was generated with all the variables (Appendices 1 & 2) in order to proceed to its statistical and epidemiological analysis, choosing the right tests according to the variable type. In order to process the variables used in the questionnaires with the software SPSS 15.0 for Windows (Chicago, USA), they were given a code number. Aunivariate description of all the variables liableto indicate a variation in the environment or the studied population was performed through the calculation of means and standard deviations, or relative frequencies (percentages) and their 95% confidence intervals, using the software WinEpi.8 The appropriate null hypotheses were then formulated; in all cases, they were rejected when p-values were lower than 0.050. Depending on the type of variable, the following non-parametric tests used were: Kruskal-Wallis, Mann-Whitney and Chi-square (χ2) or Likelihood Ratio (LR). With them, we could assess any association of host and environmental factors with parasite infection and also decide if the prevalence of exposed and non-exposed to a certain factor was significantly different.
Epidemiological analysis: In order to quantify the risk of infection by any of the isolated parasites whenever the fish was exposed to the Risk Factor, the Odds Ratio (OR) values were calculated using the epidemiology software Win Episcope 2.0.19 When the value of OR was higher than 1, and was not included within the interval of 95% confidence limits (IC95%), this means that it acted as a risk factor, and the likelihood of infection is as high as the value itself.
Physicochemical and microbiological quality of the water
The results recorded for both the physicochemical and the microbiological water parameters (Table 1 & 2) measured during the different studied seasons did not show any significant variations depending on the season. With a quick look on these values shown in Table 1 & 2 and the recommended values for cyprinid farming shown in Appendix 3, one can see that temperature, DO, NO2– and NH4+ values fell within the recommended range, while the pH values were always above the maximum level which may have a negative influence on the health status of the fish.
|
Water quality parameter |
Standard value for Cyprinids |
|
Temperature |
Maximum: 28ºC (= maximum temperature allowed at the limit of the mixing zone for more than 98% of the time) |
|
pH |
Range: 6-9 |
|
Nitrites |
Maximum: 0.03 mg/L NO2- (reference value) |
|
Total Ammonium |
Maximum: 0.2 mg/L NH4+ (reference value) |
|
Dissolved Oxygen (DO) |
Minimum (50% of the water samples): 8 mg/L O2 |
Appendix 3 Parameters of water quality for cyprinids, according to the Freshwater Fish Directive of the European Economic Community, EEC, 1978
NO2-: Nitrites (mg/L); NH3: Total Ammonium (mg/L); DO: Dissolved Oxygen (mg/L)
|
|
Spring (N=1) |
Summer (N=2) |
Autumn (N=2) |
Pkw |
Total (N=5) |
|
T |
19.00 |
21.25 ± 5.30 |
7.10 ± 1.27 |
0.223 |
15.14 ± 7.88 |
|
DO |
11.80 |
8.55 ± 0.77 |
14.05 ± 1.06 |
0.165 |
11.4 ± 2.84 |
|
pH |
10.37 |
9.55 ± 0.13 |
9.31 ± 1.23 |
0.368 |
9.62 ± 0.76 |
|
Cond |
167.00 |
206.50 ± 23.34 |
295.00 ± 55.15 |
0.165 |
234.00 ± 65.25 |
|
Turb |
4.56 |
4.07 ± 3.06 |
7.25 ± 3.85 |
0.819 |
5.44 ± 2.97 |
|
Alk |
85.40 |
109.80 ± 17.25 |
164.70 ± 77.64 |
0.301 |
126.88 ± 53.60 |
|
Hard |
72.00 |
99.00 ± 12.73 |
162.00 ± 50.91 |
0.165 |
118.80 ± 48.63 |
|
NH4+ |
0.050 |
0.125 ± 0.106 |
0.050 ± 0.000 |
0.472 |
0.08 ± 0.067 |
|
NO2- |
0.020 |
0.020 ± 0.014 |
0.015 ± 0.007 |
0.823 |
0.018 ± 0.008 |
|
NO3– |
2.00 |
3.50 ± 2.12 |
2.00 ± 0.00 |
0.472 |
2.60 ± 1.34 |
|
PO4 |
0.80 |
0.55 ± 0,35 |
0.45 ± 0.35 |
0.398 |
0.56 ± 0.29 |
Table 1 Physicochemical water quality at the fish farm by season
pKW: p-Values According to Kruskal-Wallis test; T: Temperature (ᵒC); DO: Dissolved Oxygen (ppm); pH; Cond: Conductivity (µS.cm-1); Turb: Turbidity (FTU); Alk: Alkalinity (mg/L); Hard: Hardness (mg/L); NH4+: Ammonium (mg/L); NO2-: Nitrites (mg/L); NO3–: Nitrates (mg/L); PO4: Phosphates (mg/L)
|
|
Spring (N=1) |
Summer (N=2) |
Autumn (N=2) |
Pkw |
Total (N=5) |
|
TA 22ºC |
484.0 |
257.5 ± 292.0 |
533.0 ± 202.2 |
0.497 |
413.0 ± 228.3 |
|
TA 37ºC |
9.0 |
187.0 ± 97.6 |
25.5 ± 6.4 |
0.165 |
86.8 ± 104.0 |
|
TC |
6.0 |
101.0 ± 140.0 |
5.0 ± 0.0 |
0.729 |
43.6 ± 87.4 |
|
FC |
0.0 |
80.0 ± 113.1 |
2.0 ± 2.8 |
0.687 |
32.8 ± 71.1 |
|
FS |
9.0 |
101.0 ± 125.9 |
38.5 ± 30.4 |
0.368 |
57.6 ± 76.9 |
|
C |
0.0 |
0.0 ± 0.0 |
0.0 ± 0.7 |
0.472 |
0.2 ± 0.5 |
Table 2 Microbiological water quality at the fish farm by season
pKW: p-Values According to Kruskal-Wallis test; TA 22ᵒC: Total Aerobes at 22ᵒC (CFU/100 mL); TA 37ᵒC: Total Aerobes at 37ᵒC (CFU/100 mL); TC: Total Coliforms (CFU/100 mL); FC: Faecal Coliforms (CFU/100 mL); FS: Faecal Streptococci (CFU/100 mL); C: Clostridia (CFU/20 mL)
Biometric parameters of the fish
From Table 3, one can see that the spawning season for this species was during the spring, when significantly the biggest and the heaviest fish were captured, measured and analyzed.
|
Fish – tench |
N |
Total Length (cm) |
Weight (g) |
|
Spawning |
10 |
20.70 ± 6.37 |
141,3 ± 98,21 |
|
Non-Spawning |
104 |
12.25 ± 2.80 |
27,02 ± 15,27 |
|
pMW |
|
<0.001 |
<0.001 |
|
Spring |
10 |
20.70 ± 6.37 |
141.30 ± 98.21 |
|
Summer |
55 |
11.70 ± 2.86 |
26.55 ± 13.64 |
|
Autumn |
49 |
12.87 ± 2.63 |
27.55 ± 17.04 |
|
pKW |
|
<0.001 |
<0.001 |
Table 3 Biometric parameters of the fish by reproductive state (spawning) and season
pMW: p-Values According to Mann-Whitney test; pKW: p-Values According to Kruskal-Wallis test
Parasitization
In total, four taxonomic groups were identified: two ciliate protozoa (Ichthyophthirius multifiliis and Tripartiella sp.), one myxozoan (Myxobolus sp.) and one monogenean (Gyrodactylus sp.). The results significantly showed that the most prevalent within the fish population were the ciliates, with 100% of it infected by trichodinids and 83.67% infected by I.multifiliis; only 5.56% was infected by the myxozoan and 2.63% by the monogenean. The intensities were only high in the case of Tripartiella sp. (+3 in 82.6% of the fish) and I.multifiliis (+2 in 82.2% of them). In the present study, no mortality was registered and the only symptom observed on the fish was an abundance of white mucus on the gills and body surface of the tenchs especially when the parasite load was high. The analysis also showed statistically significant associations between several factors and parasite abundance, as described below.
Ichthyophthirius multifiliis (“ich”): The trophonts of ich were mainly found on the gills (96.77% of the cases) and skin/gills (3.23%). Some authors20 state that the development of this phase of the parasite on the gills may occasionally have lethal effects on fish health. In our study, we noticed that the I. multifiliis abundance was significantly associated with season, with the highest intensities appearing in summer. In this season, up to 82.2% of the analyzed fish showed a level +2 (medium), dropping down to 14% in autumn and spring (Figure 2). Our results are in accordance with other authors’21,22 who state that Ichthyophthiriasis outbreaks normally occur during the warmer seasons of the year as the water temperature rises. The highest prevalence (%) was recorded in the autumn (83.67% of the fish were parasitized), when temperatures dropped down again (Table 4). According to other authors [23], very high temperatures (>28ᵒC) are lethal for ich, but as they reach their lowest values I. multifiliis seem to “hibernate” on the body of the host. In temperate regions autumn is also a season of abrupt changes, situation which may induce stress and a lower immune response in the fish, hence increasing their susceptibility to parasitization.
|
|
Spring |
Summer |
Autumn |
Total |
pX2 |
|
Prevalence (%) |
70.00 |
81.82 |
83.67 |
81.58 |
0.595 |
Table 4 Infection prevalence (%) by I. multifiliis by season
pX2: p-Value According to Chi-square (χ2) test
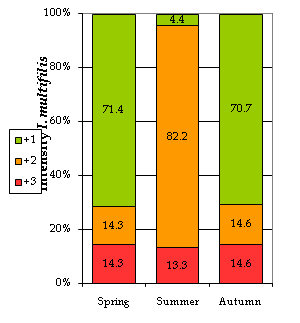
Figure 2 Infection intensities by I. multifiliis by season. +1, low intensity; +2, medium intensity; +3, high intensity; the numbers show the percentage of fish affected with particular intensity.
Tripartiellasp.: The parasite was only found on the gills of the tenchs (Figure 3). Authors23-25 mentioned that this is the preferential place for the trichodinids, though they may also be present on the skin of recently hatched larvae.26 Some intrinsic factors seemed to be linked significantly to the presence of the ciliate. In the first place, the condition factor (K) was significantly lower in the case of the infected fish (Table 5). According to authors,23,27 weakened and stressed fish are the most susceptible to the infection, especially under culturing conditions;17 though the situation may also be explained the other way around, with a high load of parasites on the fish surface leading to a lower somatic condition. Secondly, the smaller (<13 cm) and younger fish were around 15 times more likely to be infected by the ciliate (Table 6) i.e., both fish size and/or age could act as a risk factor associated with the presence of the parasite. This finding agrees with authors17,26 who mentioned that the Trichodinasis is more frequent among the larvae and juveniles.
|
|
Infected Fish (g/cm3) |
Non-Infected Fish (g/cm3) |
pMW |
|
Condition Factor |
1.47 ± 0.34 |
1.54 ± 1.20 |
<0.001 |
Table 5 Condition factor of the fish infected and non-infected fish by Tripartiella sp
pMW: p-Value According to Mann-Whitney test
|
Fish size |
≤ 13 cm |
> 13 cm |
pX2 |
OR |
|
Prevalence (%) |
84.72 |
26.19 |
0.008 |
15.630 |
Table 6 Size of the fish as risk factor associated with the presence of Tripartiella sp. in the fish farm
pX2: p-Value According to Chi-square (χ2) test; OR: Odds Ratio

Figure 3 Tripartiella sp. from the gills of the tenchs. Peristomal (upper photo) and anti-apical zone (lower photo).
Some extrinsic or environmental factors, like season and water quality, showed a significant effect on the presence of the parasite. Table 7 and Figure 4 show that both the prevalence and the intensity of infection reached their highest peaks during the summer season, significantly increasing the likelihood of parasitization by almost 6.5 times (Table 8). This could be attributed to the fish behavior that is conditioned by the temperature fluctuations. This finding agrees with author28 who mentioned that the multiplication of this parasite may be triggered by a higher activity of the fish. Other authors5,17,29 related the multiplication of this type of parasite to the more frequent fish contact in shallower water as well as the higher levels of stress during spawning activities which also lead to a weakening in their health status.30,31
|
Season |
Spring |
Summer |
Autumn |
Total |
pX2 |
|
Prevalence (%) |
0.00 |
83.64 |
53.06 |
63.16 |
<0.001 |
Table 7 Infection prevalence (%) by Tripartiella sp. by season
pX2: p-Value According to Chi-square (χ2) test
|
Season |
Summer |
Rest of the Seasons |
pX2 |
OR |
|
Prevalence (%) |
83.64 |
44.07 |
0.001 |
6.487 |
Table 8 Seasonality as risk factor associated with the presence of Tripartiella sp. in the fish farm
pX2: p-Value According to Chi-square (χ2) Test; OR: Odds Ratio
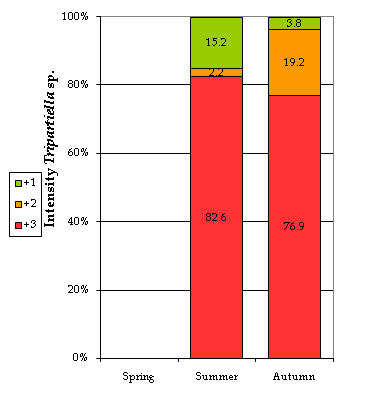
Figure 4 Infection intensities by Tripartiella sp. by season. +1, low intensity; +2, medium intensity; +3, high intensity; the numbers show the percentage of fish affected with particular intensity.
Finally, according to the results shown in Table 9, some of the studied physicochemical (hardness >100 mg/L, nitrates >3 mg/L & phosphates >0.6 mg/L) and microbiological (total coliforms >100 CFU/100mL, faecal coliforms >0 CFU/100mL, faecal streptococci >50 CFU/100mL & anaerobic clostridia >0 CFU/100mL) water parameters seemed also to be acting as risk factors for the presence of Tripartiella sp. at the fish farm. Other authors [25] point out that poor environmental quality conditions may have an influence on the presence of the parasite. In addition to their negative influence on the fish health, high pH values recorded during the three studied seasons (Table 1) (which have a reflection on the water hardness) may cause an erosion on the fish body surface, with hyper-secretion of mucus as well as cutaneous bleeding and osmoregulation unbalance,32-35 generating chronic-stress29 that can lead to immunosuppression23,36 and thus to a higher susceptibility to parasites.37
|
|
Exposed |
Non-Exposed |
pX2 |
OR |
|
|
Hardness >100 mg/L |
Hardness ≤100 mg/L |
|
|
|
Prevalence (%) |
74.16 |
24.00 |
<0.001 |
9.087 |
|
|
Nitrates >3 mg/L |
Nitrates ≤3 mg/L |
|
|
|
Prevalence (%) |
100.00 |
43,24 |
<0.001 |
529.900* |
|
|
Phosphates >0.6 mg/L |
Phosphates ≤0.6 mg/L |
|
|
|
Prevalence (%) |
83.54 |
17.14 |
<0.001 |
24.540 |
|
|
TC >100 CFU/100mL |
TC ≤100 CFU/100mL |
|
|
|
Prevalence (%) |
100.00 |
43.24 |
<0.001 |
529.900* |
|
|
FC>0 CFU/100mL |
FC ≤0 CFU/100mL |
|
|
|
Prevalence (%) |
96.65 |
13.33 |
<0.001 |
143.000 |
|
|
FS >50 CFU/100mL |
FS ≤50 CFU/100mL |
|
|
|
Prevalence (%) |
95.65 |
13.33 |
<0.001 |
143.000 |
|
|
C >0 CFU/20mL |
C ≤0 CFU/20mL |
|
|
|
Prevalence (%) |
89.66 |
54.12 |
<0.001 |
7.348 |
Table 9 Physicochemical and microbiological water quality as risk factor associated with the presence of Tripartiella sp
*Null data: Approximate Calculations (WinEpi); pX2: p-Value According to Chi-square (χ2) test; OR: Odds Ratio; TC: Total Coliforms; FC: Faecal Coliforms; FS: Faecal Streptococci; C: Clostridia
Myxobolussp.: The myxozoan was only present in the fish internal organs (liver, kidney and gonads). According to authors,26 some species of the genus Myxobolus seem to be specific to certain organs. It has also been found on the skin, gills and fins.23 Table 10 indicates that only the females were infected by Myxobolus sp. (prevalence of 5.56%). This may be related to their weakening after the spawning season.30,31 From Table 11, one can see that the parasite was only present in the summer (prevalence of 5.45%). Some authors38 stated that some myxosporidia cause epizootic diseases only during the warmer seasons of the year.
|
Sex |
Male |
Female |
Total |
pLR |
|
Prevalence (%) |
0.00 |
5.56 |
3.33 |
0.077 |
Table 10 Infection prevalence (%) by Myxobolus sp. by sex
pLR: p-Value According to Likelihood Ratio
|
Season |
Spring |
Summer |
Autumn |
Total |
pLR |
|
Prevalence (%) |
0.00 |
5.45 |
0.00 |
2.63 |
0.108 |
Table 11 Infection prevalence (%) by Myxobolus sp. by season
pLR: p-Value According to Likelihood Ratio
Gyrodactylus sp.: The metazoan was found on the fish skin (66.67% of the cases) and the gills (33.33%). Though most species seem to be able to move actively around the host surfaces,39,40 where they feed on epithelial cells, mucus and blood.17,41 However, under situations of intense infection, some species may also be found in the mouth and pharyngeal cavities.42 In general, 2.63% of the tenchs were infected, reaching 5.45% in the summer (Table 12) probably due to rising temperatures.43-45 Besides the low prevalence, also intensity was low in 100% of the cases.
|
Season |
Spring |
Summer |
Autumn |
Total |
pLR |
|
Prevalence (%) |
0.00 |
5.45 |
0.00 |
2.63 |
0.108 |
Table 12 Infection prevalence (%) by Gyrodactylus sp.by season
pLR: p-Value According to Likelihood Ratio
At the current study, the influence of host and culture conditions on the epidemiology of the tench parasites was assessed. According to the present results, the three intrinsic parameters (Fish size, somatic condition and sex), season and environmental water quality are the main risk factors that may increase, under culture conditions, the risk of presence and abundance of certain fish parasites at the fish farm. The four taxonomic groups identified along the study have a common characteristic: they are parasites with a direct life cycle. This means that they are the first to colonize the fish, especially the younger and weaker ones, and also to multiply faster whenever an abrupt change in the culture conditions occurs. In general, we can state that the life cycles and strategies of the studied tench populations, as well as the climatic conditions that define the NW-region of Spain (with seasonality as the main factor) have an impact on the dynamic balance of the host-parasite relationship. The seasonal fluctuations (especially temperature) with an alternation of environmental stress and bounty are finally reflected on the low and high levels of parasitization, respectively, both in the fish (intensity) and the fish populations (prevalence). As we have registered, the development, reproduction and transmission of the parasites are triggered under the most stable conditions of the ecosystem (hydrological, physicochemical and biological), i.e. the warmer periods of the year, when the cyprinids display a more active and gregarious behavior.
We can also state that slight modifications in the regular and “normal” conditions of these aquatic ecosystems, either by natural causes or by human intervention (e.g., high water pH), can trigger the development of one or several life cycle stages of the parasites, affecting the host-parasite balance, and causing an increase in intensity and/or prevalence of the parasites in the fish populations. Finally, the use of statistical and epidemiological analysis, like Odds Ratio, allowed the quantification of the risk and the extent at which certain risk factors were associated with the presence of the parasites. The results presented here will also help to understand the epidemiology of cultured fish, to produce estimates of expected levels of infection in populations based on the presence or absence of risk factors, and to quantify the impact that parasite diseases may have on susceptible populations.
The author is grateful to staff from Castile-León Regional Government (Junta de Castilla y León) and the University of Zaragoza, Veterinary Faculty, for their technical assistance.
None.

©2015 Illán. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.