Journal of
eISSN: 2378-3184


Letter to Editor Volume 13 Issue 3
1Department of Fisheries Technology, Bangladesh Agricultural University, Bangladesh
2Department of Surgery and Obstetrics, Bangladesh Agricultural University, Bangladesh
3Department of Microbiology and Hygiene, Bangladesh Agricultural University, Bangladesh
Correspondence: Md. Shaheed Reza, Department of Fisheries Technology, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
Received: August 27, 2024 | Published: September 9, 2024
Citation: Reza S, Alam M, Khan MFR, et al. Phage therapy to combat antibiotic resistance in aquaculture. J Aquac Mar Biol. 2024;13(3):104-106. DOI: 10.15406/jamb.2024.13.00403
Recently, antimicrobial resistance has become a major public health concern as it threatens the effective prevention and treatment of an ever-increasing range of infections caused by pathogens particularly bacteria. Phage therapy, the use of bacteriophages to target and destroy specific bacterial pathogens, has emerged as a promising alternative to antibiotics in aquaculture especially for combating antibiotic-resistant strains. This approach offers a targeted approach by employing naturally occurring bacteriophages that specifically infect and lyse bacterial pathogens without disrupting beneficial microflora. Recent studies have demonstrated the efficacy of phage therapy in reducing bacterial infections such as Vibrio and Aeromonas in various aquaculture environments. This abstract highlights the advantages of phage therapy in addressing antibiotic resistance in aquaculture, outlines the steps for its implementation and discusses the associated challenges.
Keywords: aquaculture, bacteriophages, bacterial pathogen, efficacy, lytic enzymes
Recently, the prevalence of antimicrobial resistance (AMR) among natural and commensal bacteria has increased which poses a threat to public health. This increase is largely driven by the overuse, misuse and improper disposal of antibiotics1,2 leading to the frequent detection of antibiotic resistance genes (ARGs) in aquaculture systems worldwide.3,4 This occurrence of ARGs in fish farms highlights the inefficiency of antibiotic treatments in in these environments.5 The World Health Organization (WHO) has identified ARGs and antibiotic-resistant bacteria as major threats to public health in the 21st century with the spread of ARGs in aquatic ecosystems becoming a growing concern.4 ARGs can enter the food chain through aquaculture products due to uncontrolled prophylactic and therapeutic use of antibiotics in fish diets. Additionally, fecal waste from farmed fish contributes to the enrichment of ARGs in sediments, potentially spreading them to the surrounding environment. This is not limited to freshwater aquaculture but also affects mariculture in both developed and developing countries. The rise in multidrug-resistant bacteria such as Aeromonas, Streptococcus, Edwardsiella, Vibrio, Staphlylococcus further emphasizes the urgent need to address antibiotic resistance in aquaculture. Several environmental alternatives have been proposed to combat bacterial diseases such as probiotics, vaccines and bacteriophages.6,7 However, Fowler et al.8 reported that the challenges related to the colonization of probiotics and vaccine administration have hindered their application in aquaculture.
In contrast, bacteriophages have been favored for their high specificity, lack of drug residues, and ease of application.9 They can combat antibiotic resistance in bacterial pathogens by:
Bacteriophages are bacteria-specific viruses that multiply inside the resident bacteria by making use of some / all of the host biosynthetic machinery and, as such, are not pathogenic for animals, including humans.10 These are relatively simple viruses that target a bacterial host are widely available in soil and human and animal guts. Some of the bacteriophages are used to treat bacterial diseases in humans and farm animals.11 Research showed that it can control bacterial infection in shrimp.12,13 It was also reported that a phage lysate of Aeromonas hydrophila induced a high immune response in Cyprinus carpio compared to a formalin-killed vaccine.14 Therefore, bacteriophages may be considered as an alternative to antibiotics in aquaculture for increased fish production as evidence shows that the applications of a single or mixture of specific bacteriophages by muscle injection or drinking water15 to animals reduced bacterial infection and mortality.16
The use of phages in aquaculture involves several steps to ensure their effectiveness and safety (Figure 1). First, specific bacteriophages that target the pathogenic bacteria of concern in the aquaculture environment must be isolated and identified. This involves sampling of infected fish tissues to identify the bacterial pathogen. Here emphasis is given to identify and quantify cultivable antibiotic-resistant bacteria of interest. Then phages are screened that can effectively infect and lyse these bacteria. Once isolated, the selected phages undergo extensive characterization to determine their host range, specificity and lytic activity to ensure that they target only the harmful bacteria without disrupting beneficial microbiota. Following this, the phages are amplified and purified to produce sufficient quantities for application. Safety assessments are conducted to confirm that the phages do not carry any harmful genes such as those coding for toxins or antibiotic resistance and to evaluate their potential impact on non-target organisms and the environment. Field trials are then conducted to assess the efficacy of phage treatment in real-world aquaculture settings. These trials involve administering the phages to infected fish populations through various delivery methods such as in feed, water, or direct injection while monitoring the health of the fish, bacterial load and environmental parameters. The data collected from these trials are analyzed to optimize dosing strategies, timing and application methods for maximum effectiveness.
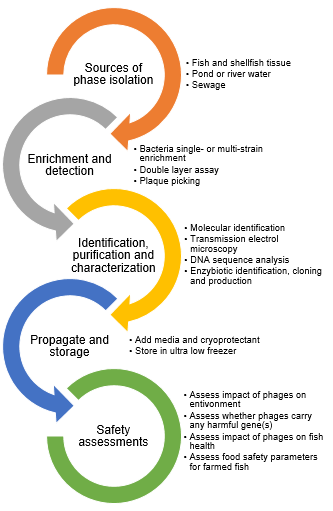
Figure 1 Steps involved in screening and application of phage in aquaculture.13
There are several challenges to phage therapy application in aquaculture. One of the primary concerns is the potential development of phage-resistant bacterial strains that can undermine the long-term efficacy of the treatment. This necessitates continuous monitoring and the potential need for phage cocktails to combat resistance.17,18 The phage specificity can also be a limitation as a broad range of phages may be required to cover all possible bacterial threats in diverse aquaculture environments. Additionally, the environmental stability of phages is a challenge. A wide variety of water quality parameters such as temperature, pH and salinity can affect their survival and effectiveness. Regulatory challenges also pose a significant barrier the approval process for phage therapy in aquaculture can be complex and time-consuming. This may require rigorous safety assessments and compliance with varying national and international standards. Finally, the acceptance of phage therapy among aqua farmers and consumers presents a potential hurdle as public perception and understanding of phage technology are still evolving. Addressing these challenges is crucial for integrating phage therapy as a viable alternative to antibiotic treatment in aquaculture.
None.
The author declares that there are no conflicts of interest.

©2024 Reza, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.