Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 2
1Department of Fisheries Management, Bangladesh Agricultural University, Bangladesh
2Department of Arts, Education & AgriTech, Melbourne Polytechnic, Australia
3Center for Water and Environment, Research Institute, King Fahd University of Petroleum & Minerals, Saudi Arabia
Correspondence: Saleha Khan, Department of Fisheries Management, Bangladesh Agricultural University Mymensingh -2202, Bangladesh
Received: May 02, 2024 | Published: June 18, 2024
Citation: Nur AA, Ritu JR, Haque M, et al. Occurrence, abundance and seasonal distribution of benthic macro invertebrates in relation to the water quality and bottom soil properties in a fish pond. J Aquac Mar Biol. 2024;13(2):53-62. DOI: 10.15406/jamb.2024.13.00397
Benthic macroinvertebrates are essential components of aquatic biotic communities, performing several ecological roles in aquatic ecosystem functioning including nutrient circulation and recirculation. The species diversity, abundance and seasonal dynamics of benthic macroinvertebrates in a fish pond and their relation with pond bottom soil and water quality parameters were examined for a duration of one year during January to December. A total of 21 genera under four (4) different major groups of benthic invertebrates were identified. The mean abundance and a number of genera of each benthic macroinvertebrate group during the study period were in the following order: Oligochaeta (13 genera) > Chironomidae (4 genera) > Ceratopogonidae (2 genera) > Mollusca (2 genera). Among the genera, Tubifex spp., Pentaneura spp. and Culicoides spp. were most abundant and frequently occurred in each month. The interaction of the water quality parameters and soil properties played an important role in the structure of the benthic communities. The total nitrogen (%) content of the sediments was found to be positively correlated with the occurrence and abundance of Chironomidae (r = 0.764; p < 0.01). The relationship between environmental variables and assessed soil quality properties with the diversity indices showed significant variation over months, whereas the Simpson diversity index (Ds) was judged to be a lower level of contamination. The Shannon-Wiener index (H´) and Richness Index (R) and Pielou evenness (J) revealed that the benthic community distribution was uniform, and the water quality and soil parameters revealed tolerable conditions for the productivity of the pond. Considering the presence of 21 genera of macroinvertebrates, diversity index and properties of the soil and water, the fish pond can be considered as a moderately productive pond.
Keywords: macrobenthic fauna, environmental variables, soil properties, diversity indices, bio-indicator
Benthic macroinvertebrates are important organisms and indispensable parts of all aquatic ecosystem which contribute as live food and energy for fishes as well as other aquatic organisms.1 Benthic macroinvertebrates, which are assemblages of animals living in or on the bottom sediments, depend upon the decomposed organic matter for their basic food supply in any aquatic ecosystem.2,3 The benthic fauna is an important part of the aquatic ecosystem, which serves as a link between primary producers, decomposers, and higher tropic levels and plays a major role in the detritus food chain, constituting the final major trophic groups in the aquatic ecosystem.4 The presence and higher abundance of benthic macroinvertebrates indicate the biological and water quality conditions of an aquatic environment because benthic organisms depend on nutrients from phytoplankton, bacteria and detritus and facilitate biogeochemical cycles for their growth.3,5 The abundance and distribution of benthic macroinvertebrates are very commonly used in bio-monitoring because they significantly respond to organic and inorganic pollution.6 It has been reported that where benthic macroinvertebrates are enormous in number, they decrease planktonic biomass and influence its composition.7,8 Benthic macro invertebrates are an important source of amino acids, fatty acids, vitamins, and minerals for the growth of aquatic organisms, especially the fishes.9,10 The growth and abundance of benthic macroinvertebrates mostly depend on different environmental factors, especially on the chemical properties of bottom soil.11 The benthic community influences the amount of nutrients released from the bottom sediments. On the other hand, pond bottom sediments indicate the source of nutrients for bottom-dwelling organisms and other aquatic organisms as well as increase the productivity of a water body.12 Freshwater aquaculture has rapidly increased in some Asian countries including Bangladesh. In semi-intensive and intensive aquaculture practices huge quantities of supplemental feed are used, and a part of which are directly and/or indirectly deposited as organic matter on the pond bottom.13,14 These organic matters are influencing the quality of both of the water and bottom mud. For successful qualitative and quantitative fish culture and aquatic environmental management, it is important to know the occurrence, abundance, and seasonal dynamics of benthic fauna in a fish pond, which will, in turn, help to increase the production of fish.15
The present study was planned to see the influence of water quality parameters and chemical properties of pond bottom soil on the abundance and seasonal dynamics of benthic macroinvertebrates in a fish pond.
Study area
The study was carried out in a pond beside the Faculty of Fisheries, Bangladesh Agricultural University (24.726721N; 90.434871E), Mymensingh, for a duration of twelve months from January to December (Figure 1). The pond is rectangular shaped and receives water from the water supply system of the university and from rainfall.
Water quality parameters
Some water quality parameters such as temperature (ºC), dissolved oxygen (mg/l) and pH were measured fortnightly. Water temperature was recorded with a Celsius thermometer. pH and dissolved oxygen (mg/l) were measured using a digital water quality Multi Meter (HACH -HQ40d multi).
Chemical properties of pond bottom soil
Pond bottom sediment samples were collected at 15 days interval from the area of soil-water interface by an Ekman Dredge (covering a mouth area of 225 cm2) (Figure 2). Chemical properties of pond bottom soil, namely, pH, organic carbon (%), available phosphorus (ppm), total nitrogen (%) and organic matter (%) were measured following standard methods.16
Study of benthic fauna
The benthic macro invertebrate samples were collected at 15 days interval from 3 sampling sites on each sampling day between 9:00 AM and 11:00 AM for twelve consecutive months from the pond by using an Ekman dredge (covering an area of the mouth 225 cm2). After collection, the bottom sediment was drained on a fine mesh (0.2 mm) screen net and washed in water. The collected benthic macro invertebrates were preserved in 10% buffered formalin in plastic bottles.
Laboratory study
The formalin preserved macro invertebrates were transferred to a petri-dish and washed carefully with water to remove the remaining washable detritus and mud. Then, the organisms were separated from each other by using sorting needles and fine forceps. Finally, the benthic macro invertebrates were identified under a magnifying glass or a stereo microscope17–20 and counted according to different taxonomic position.
Calculation of benthic fauna
Benthic macro invertebrate population density (no. per m2) was determined following Rahman.21
Benthic invertebrate population density (no. per m2)
= number found in pond bottom soil collected by the Ekman Dredge × 44.44
= number per 225 cm2 × 44.44
Where, area of the open mouth of the Ekman dredge = 225 cm2
Benthic community analysis
A number of diversity indices were used to understand the diversity, species richness and Pielou evenness of the benthic invertebrates of the fish pond. The Simpson diversity index (Ds),22 Shannon-Wiener index (H´),23 Margalef’s richness index (R),24 and Pielou evenness (J)25 were calculated using the quantitative data (per m2).
Simpson diversity index,
Where,
= Simpson’s index of diversity,
= number of all individuals,
= number of individuals of a particular species.
Shannon-Wiener index,
Where,
= species diversity,
= total number of individuals in the sample,
= number of individuals in each species.
Margalef’s richness index,
Where,
= total population across all species,
= richness of species,
= number of species.
Pielou evenness,
Where,
= the Shannon-Wiener index,
= total species number,
= Pielou evenness.
Multi-variate analysis
Pearson26 introduced the principal component analysis which is a type of multi-variate study. This analysis transforms the data through linear transformation into a new coordinate system. In the new system, the first principal component obtained through projections of the data explains the greatest variance. The other descending components will represent gradually decreasing variances. Based on the contributions of different variables on the principal components, the similarity among different variables can be established. The major steps of PCA includes: (i) determination of standard score, (ii) calculation of co-variance matrix, (iii) computation of the eigenvectors and eigenvalues, and (iv) identification of the principal components. The PCA determines the most important features from the dataset, reduces data size, and reveals the structure of the variables or the observations.27
Hierarchical clustering analysis
The unsupervised clustering analysis based on hierarchical clustering is typically performed to determine groups in the dataset based on the similarity among variables. The clustering algorithm ensures the compactness of variables in clusters and dissimilarities among clusters. A hierarchical clustering algorithm can be implemented by adopting an agglomerative28 or divisive clustering algorithm.29 The agglomerative approach relies on the bottom-up approach by considering each variable as a cluster. In each step, the most heterogeneous cluster is turned into two. The process will continue unless all the variables are assigned to clusters. The hierarchical clustering algorithm based on a top-down approach. The tree-based structure of the variables obtained through a hierarchy clustering algorithm is known as a dendrogram. The height in a dendrogram represents the similarity or dissimilarity between variables. Different distance measures can be selected to determine the distance between variables.
Data analysis
The SPSS (Statistical Package for Social Science) version 20 was used to analyze the data. Values are expressed as mean ± standard error of the mean (SEM). A one-way ANOVA (Analysis of Variance) followed by DMRT (Duncan’s Multiple Range Test) was performed at 1% level of significance. Linear correlation between the abundance of different groups of benthic macro invertebrates and measured water quality and pond bottom soil properties was analyzed both at 1% and 5% level of significance.
Water quality parameters
Monthly variations of some physico-chemical parameters of water of the studied pond such as water temperature (°C), dissolved oxygen (mg/l) and pH were measured throughout the study period (Table 1). The water temperature varied from 20.5 ± 0.5 °C to 30.6 ± 0.9 °C. The mean (± SEM) water temperature was highest (30.6 ± 0.9 °C) in October and lowest (20.5 ± 0.5 °C) in February. The dissolved oxygen was found to be variable and ranged from 4.7 ± 0.5 to 9.4 ± 0.8 mg/l. The highest DO was recorded as 9.4 ± 0.8 mg/l in February and the lowest was 4.7 ± 0.5 mg/l in December. pH ranged from 8.1 ± 0.1 to 8.7 ± 0.1 with the highest (8.7 ± 0.1) in February and the lowest (8.1 ± 0.1) in July.
|
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
|
Temp (ºC) |
20.6 ±0.1 |
20.5 ±0.5 |
23.7 ±0.9 |
27.3 ±1.3 |
29.4 ±1.4 |
29.2 ±0.1 |
28.9 ±0.2 |
29.5 ±0.6 |
30.2 ±0.4 |
30.6 ±0.9 |
25.8 ±1.3 |
21 ±0.8 |
|
pH |
8.5 ±0.1 |
8.7 ±0.1 |
8.5 ±0.1 |
8.5 ±0.1 |
8.7 ±0 |
8.3 ±0 |
8.1 ±0.1 |
8.1 ±0.1 |
8.3 ±0.1 |
8.2 ±0 |
8.3 ±0 |
8.3 ±0.2 |
|
DO (mg/l) |
4.9 ±0.4 |
9.4 ±0.8 |
9 ±0.1 |
8.4 ±1.1 |
7.7 ±0.1 |
7.2 ±0 |
7.4 ±0.1 |
8.4 ±0.4 |
8 ±0.1 |
6.1 ±0.1 |
4.8 ±0.2 |
4.7 ±0.5 |
Table 1 Monthly mean (± SEM; n = 6) values of measured water quality parameters
Chemical properties of pond bottom-soil
Monthly variations of some chemical parameters of pond bottom soil of the studied pond such as pH, organic carbon (%), total nitrogen (%), organic matter (%) and available phosphorus (ppm) were analyzed throughout the study period (Table 2). The soil pH fluctuated from 7.6 ± 0 to 8.6 ± 0.1 and the highest was found in April and the lowest in December. The mean (± SEM) value of organic carbon was found highest (1.80 ± 0.18 %) in April and lowest (0.86 ± 0.02 %) in September. The mean (± SEM) value of total nitrogen ranged from 0.08 ± 0 % to 0.25 ± 0.01 %. Organic matter was found highest (3.12 ± 0.3 %) in April and lowest (1.47 ± 0.04 %) in September. Available phosphorus in soil was found highest (43.49 ± 6.42 ppm) in April and lowest (19.03 ± 0.06 ppm) in September.
|
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
|
Soil pH |
7.7 ±0.1 |
7.7 ±0 |
7.7 ±0.2 |
8.6 ±0.1 |
8.1 ±0.2 |
8 ±0.1 |
8 ±0.2 |
8.3 ±0 |
8.3 ±0.1 |
8.2 ±0 |
7.8 ±0.1 |
7.6 ±0 |
|
Organic carbon (%) |
1.5 ±0.13 |
1.26 ±0.04 |
1.53 ±0.15 |
1.8 ±0.18 |
1.51 ±0.06 |
1.79 ±0.07 |
1.69 ±0.05 |
1.48 ±0.02 |
0.86 ±0.02 |
1.11 ±0.16 |
1.56 ±0.03 |
1.49 ±0.04 |
|
Total N (%) |
0.16 ±0.02 |
0.12 ±0.01 |
0.17 ±0.03 |
0.25 ±0.01 |
0.17 ±0.01 |
0.2 ±0.01 |
0.13 ±0.01 |
0.1 ±0.01 |
0.08 ±0 |
0.1 ±0.01 |
0.2 ±0.01 |
0.16 ±0.01 |
|
Available P (ppm) |
24.24 ±1.23 |
23.54 ±1.84 |
31.98 ±4.12 |
43.49 ±6.42 |
27.26 ±0.94 |
31.72 ±1.43 |
28.7 ±1.18 |
30.25 ±3.25 |
19.03 ±0.06 |
23.01 ±2.67 |
29.44 ±2.85 |
21.45 ±1.44 |
|
Organic matter (%) |
2.59±0.23 |
2.17±0.07 |
2.59 ±0.3 |
3.12 ±0.3 |
2.6 ±0.11 |
3.07 ±0.11 |
2.91 ±0.09 |
2.54 ±0.67 |
1.47 ±0.04 |
1.92 ±0.29 |
2.7 ±0.06 |
2.58 ±0.07 |
Table 2 Monthly mean (± SEM; n = 6) values of some chemical properties of pond bottom-soil
Abundance and percentage composition of benthic invertebrates
A total of 21 genera of the benthic macro invertebrates comprised of four major groups such as Oligochaeta, Chironomidae, Ceratopogonidae and Mollusca were identified in the fish pond (Table 3).
|
Groups |
Genera |
|
Oligochaeta (Annelida) |
Tubifex, Limnodrilus, Dero, Aeolosoma, Chaetogaster, Branchiura, Nais, Peloscolex, Ophidonais, Enchytraeus, Stylaria, Rhynchelmis, Pristina |
|
Ceratopogonidae (Arthropoda) |
Culicoides, Palpomyia |
|
Chironomidae (Arthropoda) |
Chironomus, Pelopia, Pentaneura, Tanypus |
|
Mollusca |
Lymnaea, Viviparous |
Table 3 List of different genera of benthic macro invertebrates found in the fish pond
Four representative genera (Branchiura, Culicoides, Chironomus and Lymnaea) from the four different groups (Oligochaeta, Ceratopogonidae, Chironomidae and Mollusca) are shown in Figure 3.

Figure 3 Benthic fauna (a: Branchiura sp., b: Culicoides sp., c: Chironomus sp. and d: Lymnaea sp.) found in the studied pond.
The annual percentage composition of the four different groups of benthic macro invertebrates found in the study is shown in Figure 4.
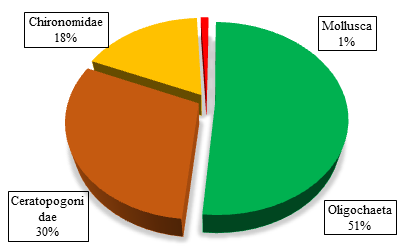
Figure 4 Percentage compositions of the different groups of benthic invertebrates in the studied pond.
Among the different groups of benthic fauna, Oligochaeta ranked top in respect of both abundance and genera throughout the study period. They represented the highest (13) number of genera and composed of 51% of the total benthic fauna (Figure 3). Ceratopogonidae represented by two (2) genera and constituted 30% of the total individuals. Chironomidae and Mollusca represented as the third and the least dominant groups of benthic fauna respectively. Chironomidae represented by four (4) genera but occupied 18% of the total individuals and Mollusca had only two (2) genera and comprised only 1% of the total individuals.
The quantitative abundance of different groups of benthic fauna in the fish pond varied with months (Figure 5).
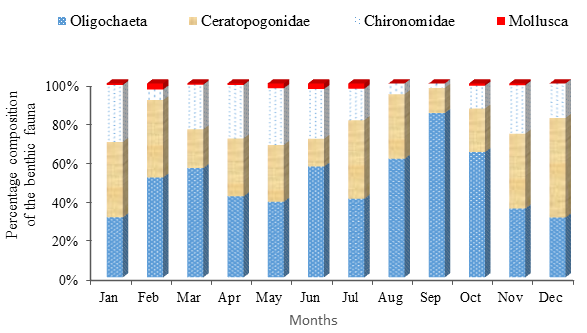
Figure 5 Percent composition of different groups of benthic macro invertebrates in different months in the fish pond.
The monthly mean (± SEM) abundance of Oligochaeta was found highest (1362.82 ± 119.19 individuals/m2) in September and lowest (222.20 ± 12.82 individuals/m2) in July. The Ceratopogonidae was found as the second dominant group of benthic fauna. Monthly mean (± SEM) abundance of Ceratopogonidae was found highest (629.56 ± 162.44 individuals/m2) in November and lowest (111.10 ± 33.94 individuals/m2) in June. The Chironomidae was found to be the third most dominant group of benthic fauna. Monthly mean (± SEM) value of Chironomidae was found highest (407.36 ± 158.33 individuals/m2) in November and lowest (37.03 ± 14.81 individuals/m2) in September. The Mollusca was found as the least dominant group of benthic fauna. The monthly mean (± SEM) abundance of Mollusca was found highest (29.62 ± 14.81 individuals/m2) in February and this group was absent in August, September and December (Table 4).
|
Groups |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
Level of sig. |
|
Oligochaeta |
288.86 ±12.82de |
488.84 ±58.78cde |
688.82 ±33.94bc |
414.77 ±109.1cde |
237.01 ±26.70e |
444.4 ±58.78cde |
222.2 ±12.82e |
407.36 ±185.1cde |
1362.82 ±119.19a |
851.76 ±48.56b |
577.72 ±188.97bcd |
333.3 ±101.82de |
* |
|
Ceratopogonidae |
362.92 ±75.16abc |
377.74 ±147.9abc |
244.42 ±44.44c |
296.26 ±60.62bc |
177.76 ±44.44c |
111.1 ±33.94c |
222.2 ±67.88c |
222.2 ±114.02c |
207.38 ±96.28c |
296.26 ±63.28bc |
629.56 ±162.44a |
555.5 ±64.14ab |
* |
|
Chironomidae |
274.04 ±19.59ab |
51.84 ±19.59b |
281.45 ±70.6ab |
274.04 ±207.7ab |
177.76 ±46.25ab |
199.98 ±46.25ab |
88.88 ±25.65b |
37.03 ±19.59b |
37.03 ±14.81b |
155.54 ±38.48ab |
407.36 ±158.33a |
192.57 ±45.05ab |
* |
|
Mollusca |
7.4 ±7.40 |
29.62 ±14.81 |
7.4 ±7.40 |
7.4 ±7.40 |
14.81 ±14.81 |
22.22 ±22.22 |
14.81 ±14.81 |
0 |
0 |
14.81 ±7.40 |
14.81 ±7.40 |
0 |
NS |
Table 4 Monthly variations in abundance (mean ± SEM; n = 6) of different groups of benthic invertebrates (unit/m2) in the studied pond
Data were taken two times a month at fortnightly interval and their mean values were taken to show the monthly variation of benthic invertebrates
*. Mean values with different superscripts (a, b, c, d and e) in the same row differed significantly (p<0.01).
NS: Mean values are not significantly different.
The monthly mean (± SEM) abundance of benthic fauna was found highest in September and lowest in July (Figure 6).
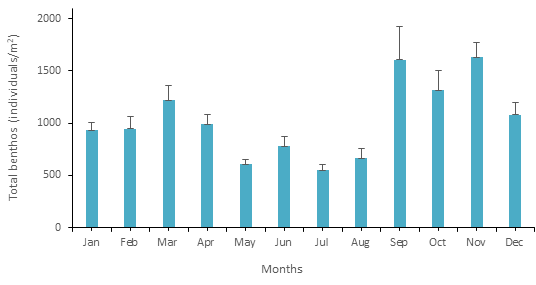
Figure 6 Monthly mean (± SEM) abundance (individuals/m2) of total benthos in the fish pond throughout the study period.
The relation between the abundance of different groups of benthic invertebrates with measured water quality and pond bottom soil properties
Correlation analysis showed a relationship between the abundance of different groups of benthic macro invertebrates and measured water quality and pond bottom soil properties (Table 5). Abundance of benthic invertebrates showed both positive and negative correlation among the measured water quality parameters and pond bottom soil properties. The group of Oligochaeta showed positive correlation between the temperature, DO and soil pH, and negative correlation with organic carbon (r = - 0.786, p<0.01) and organic matter (r = - 0.793, p<0.01). Ceratopogonidae showed negative correlation with all measured parameter and significantly negative correlation with temperature (r = - 0.598, p<0.05). Pond bottom soil properties were positively correlated with the abundance of Chironomidae. Total N showed positive correlation with the group of Chironomidae (r = 0.764, p<0.01). Mollusca showed positive correlation with pond bottom properties (organic carbon, total N, available phosphorus and organic matter) and negative correlation with temperature and soil pH.
|
Water quality properties |
Pond bottom soil properties |
|||||||
|
Benthic groups |
Temp (°C) |
pH |
DO (mg/l) |
Soil pH |
Organic Carbon (%) |
Total N (%) |
Available P (ppm) |
Organic matter (%) |
|
Oligochaeta |
0.314 |
-0.135 |
0.171 |
0.289 |
-.786** |
-0.464 |
-0.366 |
-.793** |
|
Ceratopogonidae |
-.598* |
-0.02 |
-.623* |
-0.498 |
-0.018 |
0.179 |
-0.187 |
-0.001 |
|
Chironomidae |
-0.29 |
0.126 |
-0.499 |
-0.318 |
0.488 |
.764** |
0.417 |
0.49 |
|
Mollusca |
-0.08 |
0.369 |
0.196 |
-0.263 |
0.168 |
0.182 |
0.053 |
0.166 |
|
Total benthos |
-0.068 |
-0.079 |
-0.265 |
-0.062 |
0.547 |
-0.088 |
-0.271 |
-0.545 |
Table 5 Correlation between the abundance of different groups of benthic invertebrates with measured water quality and pond bottom soil properties
Notes: n = 24, *: significance level = 0.05 (two-tailed), **: significance level = 0.01 (two-tailed).
Community structure
In the fish pond, about 21 genera belonging to four major groups of benthic macro invertebrates were found. Oligochaeta, Chironomidae and Ceratopogonidae were found frequently in the fish pond. But Ceratopogonidae was very few in number than annelids. Among Oligochaeta, Tubifex sp. was the most dominant species in the fish pond. Culicoides sp. and Chironomus sp. were the second most abundant species followed by Pentaneura, Nais and Dero (Table 6). It was observed that the genera Tubifex, Culicoides and Chironomus were important in terms of both diversity and abundance.
|
Benthic fauna |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
Branchiura sp |
* |
* |
* |
* |
- |
- |
- |
* |
* |
- |
* |
* |
|
Tubifex sp |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Limnodrilus sp |
* |
* |
- |
* |
* |
* |
- |
* |
- |
- |
* |
* |
|
Dero sp |
* |
* |
* |
* |
* |
* |
* |
- |
* |
* |
* |
* |
|
Aelosoma sp |
* |
* |
* |
* |
* |
* |
* |
- |
* |
* |
* |
* |
|
Chaetogaster sp |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
* |
* |
|
Rhynchelmis sp |
* |
* |
* |
- |
- |
- |
- |
- |
* |
* |
* |
- |
|
Nais sp |
- |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
- |
|
Peloscolex sp |
* |
* |
- |
- |
- |
* |
* |
- |
* |
* |
* |
* |
|
Enchytracus sp |
* |
* |
- |
- |
- |
* |
- |
- |
- |
- |
* |
* |
|
Ophidonais sp |
* |
* |
- |
- |
- |
- |
- |
- |
- |
- |
- |
* |
|
Stylaria sp |
* |
* |
* |
- |
- |
- |
- |
- |
- |
- |
* |
- |
|
Pristina sp |
- |
* |
- |
- |
- |
- |
* |
* |
* |
- |
- |
- |
|
Pelopia sp |
* |
* |
* |
* |
* |
* |
- |
- |
* |
* |
* |
* |
|
Pentaneura sp |
* |
* |
* |
* |
* |
* |
* |
- |
- |
- |
* |
* |
|
Chironomus sp |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Tanypus larvae |
- |
* |
* |
* |
* |
* |
* |
* |
* |
- |
- |
- |
|
Culicoides sp |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
|
Palpomyia sp |
- |
* |
* |
* |
- |
* |
* |
* |
* |
- |
* |
* |
|
Lymnaea sp |
* |
* |
* |
* |
- |
* |
* |
- |
* |
* |
* |
- |
|
Viviparous sp |
- |
* |
- |
- |
* |
- |
- |
- |
- |
- |
- |
- |
Table 6 Distribution of benthic invertebrates in the fish pond
Present (*); absent (-)
Diversity of the benthic fauna was highest in February and lowest in August in the fish pond. The distributions of benthic invertebrates, taxonomic richness and diversity were approximately symmetric in the fish pond. The monthly changes in diversity and distribution of benthic invertebrates in the fish pond are shown in Table 6.
The values of Simpson diversity index (Ds) Shannon’s diversity index (H´), Margalef’s richness index (R) and Pielou evenness (J) are given in Table 7.
|
Diversity index |
Groups |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
|
Simpson diversity index (Ds) |
Oligochaeta |
0.843 |
0.825 |
0.723 |
0.785 |
0.736 |
0.756 |
0.759 |
0.565 |
0.782 |
0.728 |
0.867 |
0.812 |
|
Chironomidae |
0.516 |
0.716 |
0.67 |
0.734 |
0.722 |
0.669 |
0.621 |
0.325 |
0.41 |
0.453 |
0.642 |
0.655 |
|
|
Ceratopogonidae |
0 |
0.069 |
0.104 |
0.377 |
0 |
0.307 |
0.446 |
0.496 |
0.219 |
0 |
0.139 |
0.054 |
|
|
Mollusca |
0 |
0.517 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Total benthos |
0.818 |
0.802 |
0.857 |
0.891 |
0.85 |
0.889 |
0.871 |
0.789 |
0.844 |
0.803 |
0.831 |
0.734 |
|
|
Shannon-Wiener |
Oligochaeta |
2 |
1.98 |
1.52 |
1.64 |
1.44 |
1.59 |
1.57 |
1.12 |
1.7 |
1.48 |
2.18 |
1.85 |
|
index (H´) |
Chironomidae |
0.87 |
1.3 |
1.23 |
1.34 |
1.32 |
1.22 |
1.01 |
0.5 |
0.73 |
0.64 |
1.06 |
1.08 |
|
Ceratopogonidae |
0 |
0.15 |
0.21 |
0.1 |
0 |
0.49 |
0.64 |
0.69 |
0.37 |
0 |
0.27 |
0.13 |
|
|
Mollusca |
0 |
0.69 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Total benthos |
2.12 |
2.17 |
2.19 |
2.36 |
2.13 |
2.36 |
2.24 |
1.79 |
2.08 |
1.83 |
2.23 |
1.87 |
|
|
Richness index (R) |
Oligochaeta |
1.48 |
1.78 |
0.959 |
0.827 |
0.77 |
1.045 |
0.914 |
0.688 |
0.987 |
0.72 |
1.555 |
1.331 |
|
Chironomidae |
0.413 |
0.668 |
0.534 |
0.6 |
0.578 |
0.57 |
0.438 |
0.276 |
0.49 |
0.263 |
0.36 |
0.413 |
|
|
Ceratopogonidae |
0 |
0.165 |
0.179 |
0.209 |
0 |
0.209 |
0.188 |
0.186 |
0.17 |
0 |
0.156 |
0.159 |
|
|
Mollusca |
0 |
0.295 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Total benthos |
2.057 |
2.744 |
1.864 |
1.833 |
1.595 |
2.009 |
1.74 |
1.255 |
1.622 |
1.242 |
2.052 |
1.86 |
|
|
Pielou evenness (J) |
Oligochaeta |
0.872 |
0.798 |
0.782 |
0.912 |
0.894 |
0.816 |
0.873 |
0.696 |
0.819 |
0.828 |
0.91 |
0.841 |
|
Chironomidae |
0.796 |
0.944 |
0.891 |
0.97 |
0.951 |
0.878 |
0.92 |
0.717 |
0.667 |
0.917 |
0.964 |
0.977 |
|
|
Ceratopogonidae |
0 |
0.223 |
0.307 |
0.223 |
0 |
0.697 |
0.918 |
0.992 |
0.543 |
0 |
0.385 |
0.184 |
|
|
Mollusca |
0 |
0.991 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
|
Total benthos |
0.784 |
0.725 |
0.83 |
0.918 |
0.887 |
0.894 |
0.81 |
0.813 |
0.813 |
0.795 |
0.827 |
0.709 |
Table 7 Diversity of different groups of benthic invertebrates
Simpson diversity index (Ds) of the total benthic invertebrates in the fish pond ranged from 0.734 to 0.891 during the study period. The highest value was observed in April and the lowest was observed in December. Shannon-Wiener index of diversity has shown variation ranged from 1.79 in August to 2.36 in June. Species richness of benthic macrofauna was highest in February and lowest in October and the values ranged from 1.242 - 2.744. Pielou evenness indices (J) ranged from 0.709 (in December) to 0.918 (in April) in the fish pond.
Multivariate analysis
To study the variances of the benthos, and water and soil quality parameters, the PCA was adopted. The Scree plot presents the variances explained by different components (Figure 7). The first nine principal components (or dimensions) are responsible for almost 99% variances in the dataset. The correlation analysis revealed that organic carbon, nitrogen, phosphorous, and organic matter influences Oligochaeta, because these parameters have significant correlations with the same principal component. The Ceratopogonidae seems to be influenced by temperature and soil pH. On the other hand, Mollusca show slight relationship with DO. However, Chironomidae appears to be unique without any significant relationship with the water or soil parameters (Figure 8 & 9). The dotted red line in the Figure 9 is the reference line which indicates the expected contribution for uniform contribution.
Hierarchical clustering analysis
The divisive hierarchical algorithm is adopted to find the clusters of the dataset. In fact, both the approaches produced similar results for the dataset of this study. In this study, Euclidian distance was considered as the distance measure and four clusters were selected. The analysis revealed that Ceratopogonidae is clustered with water pH, soil pH, and DO. The Chironomidae and Mollusca are clustered with organic carbon, organic matter, and nitrogen. On the other hand, Oligochaeta seems to be an independent cluster (Figure 10).
Successful aquaculture depends on the quality of the aquatic environment. Suitable water quality parameters are precondition for massive production of natural fish food organisms and finally fishes and other aquatic animals, such as crustaceans, mollusks etc. The abundance and species composition of different groups of benthic macro invertebrates are regulated by biotic and abiotic factors of the freshwater ecosystem.
Water quality parameters (such as temperature, pH, dissolved oxygen, alkalinity etc.) influence the growth and the survival of aquatic organisms directly or indirectly.30,31 In the present study, the water temperature varied from 20.5 ± 0.5 ºC to 30.6 ± 0.9 ºC which is favourable for survival, growth and reproduction of aquatic organisms.32,33 The concentration of dissolved oxygen (4.7 ± 0.5 to 9.4 ± 0.8 mg/l) and the values of pH (8.1 ± 0.1 to 8.7 ± 0.1) were in suitable ranges for aquatic organisms.34
The ranges of pH, total nitrogen, organic carbon, organic matter and available phosphorus of the pond bottom soil in the fish pond were also within the productive ranges for the growth of macro invertebrates. About 21 genera of macro invertebrates belonging to Oligochaeta (13 genera), Ceratopogonidae (2 genera), Chironomidae (4 genera) and Mollusca (2 genera) were identified in the present study which is similar to Saha et al.35 who recorded 23 genera belonging to 14 families in a marsh. Edokpayi et al.36 recorded 13 genera in a lagoon of Nigeria, and Esenowo and Ugwumba37 recorded 18 genera under two phyla, Mollusca and Arthropoda in Majidun River, Nigeria. Hossain38 recorded 20 taxa of macro-zoobenthos in the Meghna River estuary and among them Oligochaeta, Polychaeta and Mesogastropoda were dominant. Lower number of macro-benthic genera than the present study was also reported by Ahmed et al.,39 Khan et al.,40 Nupur et al.,41 and Sharma et al.42 in their studies in different waterbodies. Nupur et al.41 recorded 8 genera belonging to four major groups of macrobenthos named Chironomidae, Oligochaeta, Mollusca and Ceratopogonidae in aquaculture ponds. Khan et al.40 recorded 5 major groups of macro benthos named Oligochaeta, Polychaeta, Insecta, Bivalvia and Gastropoda in the Mouri River of Khulna, Bangladesh.
In this study, the dominant groups of benthic fauna were Oligochaeta> Ceratopogonidae> Chironomidae> Mollusca. Oligochaeta was found as the most dominant group of benthic fauna while Ceratopogonidae was the second dominant group followed by Chironomidae and Mollusca which are similar to the findings of Das and Islam43 who reported Oligochaeta, Chironomidae, and Mollusca as the dominant benthic fauna in tropical freshwater ponds. A similar finding was also showed by Hasan et al.44
In the present study, Oligochaeta occupied the topmost position in respect of both abundance and number of genera. The highest abundance of Oligochaeta was found in September and the lowest in May. Ceratopogonidae was the second dominant group of benthic fauna in the present study. The highest abundance of benthic fauna was found in November and the lowest in June. Chironomidae was found as the third dominant group of benthos and the highest abundance was found in November and the lowest in September. Mollusca was found as the least dominant group of benthic fauna during the study period and this group was absent in August, September and December.
The benthic invertebrates are important for enhancement of aquatic resource and playing an important role in food chain and biological purification of water. Many invertebrates feed the decaying plants and these invertebrates provide food for aquatic organisms including fishes.
In an aquatic ecosystem, benthic invertebrates have an important contribution in mixing the oxygen flow into the sediment which recycle the organic matter.45 The qualitative and quantitative abundance of benthic fauna determine the productivity of the fisheries resources. Benthic invertebrates contain a large amount of proteins, fats, waxes, starches and oils that sometimes do not exist in the supplementary feed. Some supplementary feeds contain heavy metals which are hampering fish growth and other metabolic processes, as well as may cause human cancer and other health problems when they consume contaminated fish. It would be better if we can grow fish feeding natural food.
Relationship of different groups of benthic invertebrates with physico-chemical properties of water
Linear correlation (r) existed between different groups of benthos and water quality parameters. In this study, the mean (± SEM) values of pH ranged from 8.1 ± 0.1 to 8.7 ± 0.1 that showed the alkaline nature. The values had negative correlation with the abundance and distribution of Oligochaeta (r = - 0.135) and Ceratopogonidae (r = - 0.020) but positive correlation with the abundance of Chironomidae and Mollusca. Oligochaeta showed positive correlation with the temperature, on the other hand temperature had negative correlation with the other groups of benthic invertebrates. Ceratopogonidae had negative linear correlation (p<0.05) with the concentration of dissolved oxygen (r = - 0.623). In our study, significant correlation, either positive or negative, was observed between the abundance of different groups of benthic macro invertebrates and water quality parameters, though Habib et al.9 found significant correlation only between Viviparous bengalensis and water quality parameters among the benthic invertebrate fauna they studied. The multi-variate and clustering analysis indicated relationship between Certopogonidae and physico-chemical properties of water.
Relationship of different groups of benthic invertebrates with chemical properties of pond soil
Oligochaeta showed negative correlation (p<0.01) with organic carbon (r = - 0.786) and organic matter (r = - 0.793). The amount of total N (%) had positive effect on the growth of Chironomidae but a negative impact on the growth of Oligochaeta. Chironomidae showed a positive correlation with total nitrogen (r = 0.764), organic carbon, organic matter, and available phosphorus (r = 0.417) whereas with soil pH it showed a negative correlation. The amount of total nitrogen is the most important factor for the growth of benthic invertebrates. The effect of water quality parameters and chemical properties of pond bottom soil was found to be significant on the growth and abundance of benthic fauna in the fish pond. According to the multi-variate and clustering analysis, Ceratopogonidae, Chironomidae, and Mollusca are related to the chemical properties of pond soil.
Diversity indices
Diversity indices are a synthetic assessment of biological community structure that have two features, the number of taxa (richness) and the distribution in the number of individuals among taxa (evenness).46 Many scientists expressed that as environmental quality improves there is a corresponding addition in the number of species and a corresponding addition in diversity. A very few scientists questioned about the relevance of the assumption, particularly whether it correlates to aquatic communities.47 Diversity indices originally were designed for terrestrial ecosystems by ecologists as a community structure tool, and diversity indices work well in this environment. Wilhm and Dorris23 and Wilhm48 were the first to use a diversity index along with a water quality range to measure the level of water pollution.
The Shannon, Keefe, McIntosh, and Bergersen indices exhibit rising diversity with a higher index number. In the Shannon index, a value of 3 indicates a higher diversity than a value of 0. Simpson’s index is the complement of the other three indices, with a value of 1 indicating less diversity in the sample than a value of 0.46
In our study, the value of the Simpson index ranged from 0.734 to 0.891 during the study period. The high value of indices showing high taxon richness, and high relative abundance of benthic macroinvertebrates was due to the availability of food, type of substrate, and physicochemical factors at the study site.
The Shannon-Wiener diversity index measures the number of species and the number of individuals in each species. Shannon-Wiener Index is a sensitive indicator of pollution and its values do not fluctuate very widely. A healthy benthic macroinvertebrate community should have a higher Shannon-Wiener diversity index. Shannon-Wiener index of diversity in the present study has shown a variation range of 1.791 to 2.36. Johnson and Brinkhurst49 found values ranging from 1.00 to 3.66 in their study. Montajami et al.50 reported the Shannon-Wiener index ranging from 1.3 to 2.5 in fifty polluted streams. Osborne et al.51 noticed values ranging from a minimum of 0.14 to a maximum of 2.69 whereas Godfrey52 reported the values ranging from 1.938 to 5.34. Ransom and Dorris53 made a similar investigation in their work on Keystone reservoir in the U.S.A. According to Wilhm and Dorris,23 species diversity index (H´) value ranged from >3 indicates clean water, 1.00 to 3.00 indicates moderate and <1.00 indicates the heavily polluted condition of the water. The somewhat lower values of the index of diversity during the investigation can be attributed to the residual effect of the pollutants settled at the bottom which have come from both anthropological and natural sources including surface runoff to the water body.
In our study, species richness of benthic macrofauna was highest in February and the lowest in October, and values ranged from 1.242 to 2.743. Thus, these values suggest that the pond is moderately polluted and the macrobenthic community is under stress due to natural and anthropogenic factors.
The evenness index measures the evenness or equitability of the community by scaling one of the heterogeneity measures relative to its maximal value that each species in the sample is represented by the same number of individuals. The evenness index ranged from 0 (low equitability) to 1 (high equitability). The results of total benthic fauna evenness indices ranged from 0.709 in December to 0.918 in April.
Among the four major groups of benthic invertebrates, Oligochaeta was the most diverse group followed by Chironomidae and Ceratopogonidae. The diversity index and species richness of the group of Oligochaeta were relatively high. The diversity and species abundance of the benthic macroinvertebrates of this study can be regarded as moderately low, but considering the presence of 21 genera of benthic macrofauna and the properties of soil and water quality the pond can be said as a moderately productive one. The abundance and diversity result of the benthic macroinvertebrates showed that the biodiversity is quite fluctuated. The Margalef’s richness index and Shannon-Wiener index indicated that the studied fish pond is moderately polluted.
Every organism has its role in the environment. The diversity and ampleness of benthic macroinvertebrates are related to the biotic and abiotic factors of the environment. Tolerance of aquatic organisms may vary due to abiotic factors of the surrounding environment, and it is imperative to consider suitable management to improve the ecological status. The availability of macro and meiobenthos is very much essential for maintaining the ecological balance of an environment. Pond is an important environment providing food and habitat to aquatic organisms, and helps in maintaining the aquatic food web. It is a major contributor to the local ecosystem richness and diversity of aquatic plants and organisms and also plays an important role in human food and nutrition. For developing environmental management protocols in ponds, thorough research on prevailing environmental conditions and their impact on benthic macroinvertebrates in commercial and rural aquaculture ponds are needed.
Abdullah An Nur: Investigation, Formal Analysis and writing - Original Draft
Jinnath Rehana Ritu: Investigation and Formal Data Analysis
Sadiqul Awal: Writing - Review and Editing
Md. Mahfuzul Haque: Supervision, Writing - Review and Editing
Syed Masiur Rahman: Writing - Review and Editing
Saleha Khan: Conceptualization, Methodology, Supervision, Resources, Project Administration, Fund Acquisition and Writing - Review and Editing
Ethical approval and consent to participate: The study was conducted following the guidelines of Animal Ethics Committee of the Bangladesh Agricultural University, Mymensingh, Bangladesh.
Consent for publication: Not applicable.
Availability of data and materials: The data sets used during the current study are available from the corresponding author on reasonable request.
Funding: The research was conducted under a special financial grant from the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh (Grant number BS 79/MoST, 2019-2020) which is gratefully acknowledged.
The first author is grateful to the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh for offering a fellowship during his MS studies. We are grateful to the Department of Soil Science, Bangladesh Agricultural University for allowing us in their laboratory to analyze our soil samples.
The authors declare that there is no conflict of interest.

©2024 Nur, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.