Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 3
Department of Marine Biology, University of Mazandaran, Babolsar, Iran
Correspondence: Mohammad Hossein Gorjian Arabi, Department of Marine Biology, University of Mazandaran, Babolsar, Iran
Received: September 26, 2023 | Published: October 12, 2023
Citation: Gorjian Arabi MH. Morphometric and meristic characteristics of Hemiculter leucisculus (Teleostei:Cypryniformes) population in the Haraz River Basin (Southern Caspian Sea). J Aquac Mar Biol. 2023;12(3):252-256. DOI: 10.15406/jamb.2023.12.00381
Morphometric and meristic characteristics of Hemiculter leucisculus population were studied in the Haraz River ecosystem in spring 2021, examining 27 morphometric and 10 meristic characteristics on 100 fish caught using a gillnet. According to the results, the mean coefficients of variation were 18.144% and 10.548% in females and 17.669% and 10.714% in males for morphometric and meristic characteristics, respectively. Morphometric data were standardized before analyses to reduce the error due to allometric growth. In morphometric variables, ten factors were separated, representing 73.831% of the phenotypic variations; further, three factors were selected from meristic variables, denoting 62.838% of the phenotypic variations. The results of t-test analyses showed significant differences in 3 morphometric characteristics out of 34 morphometric and meristic characteristics of male and female fish (P≤0.05). There was an overlap between the results obtained by Principal Component Analysis concerning morphometric and meristic characteristics of male and female fish, making it impossible to separate these two sexes according to the studied characteristics.
Keywords: fish carp, morphological characteristics, principal component analyze, Caspian sea
The study of morphological characteristics aimed at defining and identifying population units has a long history in the science of fish biology.1 Morphological differences are the result of both genetic and environmental factors and the interaction between the two.2 The morphological flexibility of fish allows them to respond to environmental changes through physiological and behavioral alterations that can, in turn, lead to morphometric, reproductive, and survival changes, modulating the effects of environmental changes.3,4 These morphological changes do not necessarily lead to genetic alterations of the population.5,6 Morphometric and meristic indices are widely used in the systematic study of different fish populations and the separation of fish species from each other.7 Morphometric and meristic characteristics are an effective method to identify, separate, or overlap different populations and the first step in assessing the population structure of a species.8
Hemiculter leucisculus is one of the species of carp family in Iran, with a maximum standard length of up to 175 mm. It is most abundant in the Southern Caspian Sea basin and the Anzali Lagoon. This species is non-native to Iran and belongs to freshwater fish, but it also tolerates low salinity and is often on the surface of the water. This fish feeds on phytoplankton, zooplankton, and aquatic insects, while adults feed on the eggs of other fish and even young fish, having a wide variety of food. These fish mature at the age of 2-3 years.9
This study aimed to investigate the morphological and meristic characteristics of male and female Hemiculter leucisculus in the Haraz River basin.
Fish sampling was performed from the Haraz River in the Sorkhrud region, which leads to the Caspian Sea, in spring 2021 using a gillnet (Figure 1). The studied area is located at the geographical coordinates of E: 52˚ 27́ 28̋ and N: 36˚ 40́ 38̋.
Overall, 100 Hemiculter leucisculus fish were caught, fixed in 10% formalin, and transferred to the laboratory of the Research Center for the Caspian Region, University of Mazandaran. The 27 morphometric characteristics under study (Table 1). Morphometric data were standardized by the Beacham formula before analysis. Standardizing morphometric data will reduce the changes resulting from allometric growth.10
|
CV% |
Average ± SD Min - Max |
Average ± SD Min - Max |
Variables |
||||
|
Male |
Female |
Male |
Female |
||||
|
16.485 |
16.234 |
152.515±25.143 100–190 |
143.898±23.361 100–186 |
Total length |
|||
|
15.987 |
16.488 |
137.751±22.023 90.9- 172 |
130.601±21.534 90- 170 |
Fork length |
|||
|
16.068 |
15.888 |
123.317±19.815 83.8- 155 |
116.637±18.532 81.65- 152 |
Standard length |
|||
|
13.726 |
14.799 |
28.019±3.876 19.75- 33.2 |
26.846±3.973 19.85–36 |
Head length |
|||
|
17.838 |
19.048 |
13.813±2.464 8.5–19.95 |
13.056±2.487 9.3–17.85 |
Head width |
|||
|
15.588 |
18.379 |
19.777±3.083 13.4–26.1 |
18.450±3.391 11.55–24.75 |
Head depth |
|||
|
19.266 |
19.436 |
29.455±5.675 15.6–39 |
27.948±5.432 17.65- 40 |
Max depth body |
|||
|
20.394 |
25.625 |
11.464±2.338 5.9- 15.2 |
10.584±2.703 4.2- 19.75 |
Min depth body |
|||
|
16.849 |
18.533 |
8.19±1.380 5.15–11.15 |
7.980±1.479 5–11.85 |
Snout length |
|||
|
14.788 |
15.151 |
6.647±0.983 8.8–4.4 |
6.600±1.000 4.6–8.55 |
Eye diameter |
|||
|
16.934 |
17.492 |
8.834±1.496 5.95- 11.6 |
8.495±1.486 5.35- 11 |
Distance between eyes |
|||
|
15.830 |
19.683 |
14.245±2.255 10- 18.5 |
13.453±2.648 9- 18.55 |
After-eye length |
|||
|
21.262 |
20.689 |
18.225±3.875 11.4-26.7 |
17.400±3.600 10-24.75 |
Caudal peduncle length |
|||
|
23.903 |
17.453 |
12.475±2.982 7.7–25.25 |
11.992±2.093 8.7–15.95 |
Dorsal fin length |
|||
|
18.570 |
20.891 |
18.422±3.421 11.7- 25.75 |
17.112±3.575 10.65- 26.1 |
Dorsal fin depth |
|||
|
15.656 |
15.755 |
66.887±10.472 45.1–83.2 |
63.982±10.081 47–81.7 |
Pre-dorsal length |
|||
|
20.156 |
20.035 |
46.129±9.298 29.75- 75.2 |
44.192±8.854 24.95- 59.15 |
Post-dorsal length |
|||
|
19.467 |
17.801 |
15.652±3.047 9–24 |
14.617±2.602 10.6- 20 |
Anal fin length |
|||
|
18.043 |
18.985 |
13.384±2.415 9–18 |
12.710±2.413 8.2- 18.1 |
Anal fin depth |
|||
|
17.016 |
16.159 |
90.226±15.353 43.6- 113.9 |
86.35±13.954 56.45- 114.3 |
Pre-anal length |
|||
|
21.262 |
20.656 |
18.225±3.875 11.4- 26.7 |
17.210±3.555 10- 24.75 |
Post-anal length |
|||
|
15.039 |
16.543 |
25.886±3.893 17.9- 33.7 |
24.197±4.003 17.45- 31.35 |
Pectoral fin length |
|||
|
18.260 |
17.538 |
18.362±3.353 11.7- 23.75 |
17.151±3.008 12.8- 22.5 |
Ventral fin length |
|||
|
14.495 |
15.466 |
63.137±9.152 42.6- 78.7 |
60.287±9.324 40–79 |
Pre-ventral length |
|||
|
18.737 |
17.484 |
63.268±11.855 36.45- 81.2 |
60.093±10.507 41–80.6 |
Post-ventral length |
|||
|
18.175 |
16.721 |
35.41±6.436 22.1- 49.5 |
33.956±5.678 22.65- 45 |
Pectoral-ventral length |
|||
|
17.278 |
20.962 |
31.386±5.423 18.75- 39.45 |
29.701±6.226 18.5–43.05 |
Ventral-anal length |
|||
|
CV% |
SD |
Average |
|||||
|
Male |
Female |
Male |
Female |
|
|||
|
17.669 |
18.144 |
6.865 |
6.574 |
||||
Table 1 Mean, SD, Min, Max, and CV of morphometric characteristics of male and female Hemiculter leucisculus (mm)
: Standardized values of characteristics, : The length of observed characteristics
: Average standard length for the total sample and for all regions, : Standard length of each sample, : Regression coefficient between and for each region
Mean, standard deviation, and multivariate coefficients of variation of all morphometric and meristic characteristics were calculated for morphological diversity.11
: Variance of characteristics under study, : Mean square of the same characteristics under study.
T-test was used to determine the differences between the studied sexes in each of the characteristics. The matrix relationship of morphological characteristics was examined using factor analysis and principal component analysis (PCA), identifying the main characteristics out of the ones extracted. The above calculations were performed using SPSS (26) and EXCEL statistical (2019) software.
The mean values of coefficient of variation (CV) were 18.144% and 10.584% for the female and 17.669% and 10.714% for the male Hemiculter leucisculus fish in terms of morphometric and meristic characteristics, respectively. The mean coefficient of variation of morphometric and meristic characteristics of these two sexes showed close values for both characteristics (Table 1 and 2).
|
CV% |
Average ± SD Min - Max |
Average ± SD Min - Max |
Variables |
|||
|
Male |
Female |
Male |
Female |
|||
|
20.360 |
18.268 |
2.333±0.475 2-3 |
2.195±0.401 2-3 |
Hard rays of dorsal fin |
||
|
8.958 |
7.652 |
7.166±0.642 5-8 |
7.292±0.558 6-8 |
Soft rays of dorsal fin |
||
|
19.377 |
20.328 |
2.250±0.436 2-3 |
2.317±0.471 2-3 |
Hard rays of anal fin |
||
|
6.077 |
7.060 |
11.650±0.708 11-13 |
11.756±0.830 11-13 |
Soft rays of anal fin |
||
|
8.201 |
7.055 |
15.350±1.259 14-18 |
16.341±1.153 15-18 |
Brushed gill outer |
||
|
6.041 |
6.091 |
19.283±1.165 18-21 |
19.439±1.184 18-21 |
Brushed gill inner |
||
|
4.093 |
4.813 |
52.300±2.141 49-56 |
52.024±2.504 49-56 |
Lateral line |
||
|
11.259 |
11.463 |
9.9450±1.064 8-11 |
9.561±1.096 8-11 |
Lateral line up |
||
|
20.562 |
19.873 |
2.383±0.490 2-3 |
2.536±0.504 2-3 |
Down lateral line |
||
|
2.217 |
2.878 |
36.750±0.815 35-38 |
36.512±1.051 35-38 |
Number of vertebrae |
||
|
CV% |
SD |
Average |
||||
|
Male |
Female |
Male |
Female |
|
||
|
10.714 |
10.548 |
0.919 |
0.975 |
|||
Table 2 Mean, SD, Min, Max, and CV of meristic characteristics of male and female Hemiculter leucisculus
T-test for 27 morphometric and 10 meristic characteristics of male and female fish. According to this test, male and female fish had significant differences in 3 morphologic characteristics of head depth, dorsal fin depth, and pectoral fin base length (P≤0.05), but there were no significant differences in 24 morphometric and all meristic characteristics (P>0.05) (Table 3).
|
P value |
F computational |
Characteristics examined |
|
0/05> |
0.088 |
Total length |
|
0/05> |
0.003 |
Fork length |
|
0/05> |
0.214 |
Standard length |
|
0/05> |
0.154 |
Head length |
|
0/05> |
0.136 |
Head width |
|
0/05<* |
0.448 |
Head depth |
|
0/05> |
0.059 |
Max depth body |
|
0/05> |
0.327 |
Min depth body |
|
0/05> |
0.008 |
Snout length |
|
0/05> |
0.378 |
Diameter Eye |
|
0/05> |
0.050 |
Distance between eyes |
|
0/05> |
1.548 |
After-length eye |
|
0/05> |
0.207 |
Caudal peduncle length |
|
0/05> |
1.495 |
Dorsal fin length |
|
0/05<* |
0.033 |
Dorsal fin depth |
|
0/05> |
0.001 |
Pre-dorsal length |
|
0/05> |
0.176 |
Post-dorsal length |
|
0/05> |
0.312 |
Anal fin length |
|
0/05> |
0.086 |
Anal fin height |
|
0/05> |
0.185 |
Pre- anal length |
|
0/05> |
0.285 |
Post-anal length |
|
0/05<* |
0.189 |
Pectoral fin length |
|
0/05> |
0.405 |
Ventral fin length |
|
0/05> |
0.107 |
Pre-ventral length |
|
0/05> |
0.679 |
Post-ventral length |
|
0/05> |
0.657 |
Pectoral-ventral length |
|
0/05> |
1.338 |
Ventral-anal length |
|
0/05> |
10.571 |
Hard rays of dorsal fin |
|
0/05> |
0.580 |
Soft rays of dorsal fin |
|
0/05> |
6.638 |
Hard rays of anal fin |
|
0/05> |
1.842 |
Soft rays of anal fin |
|
0/05> |
1.648 |
Brushed gill outer |
|
0/05> |
1.573 |
Brushed gill inner |
|
0/05> |
1.551 |
Lateral line |
|
0/05> |
0.071 |
Lateral line up |
|
0/05> |
1.757 |
Down lateral line |
|
0/05> |
6.141 |
Number of vertebrae |
Table 3 The results of the t-test for morphometric and meristic characteristics of male and female Hemiculter leucisculus
Linear combination of 27 morphometric and 10 meristic characteristics using Principal Component Analysis (PCA) leads to factors that show certain features of the relationship between characteristics. Hence, the higher the variance of a factor is, the higher the participation coefficient of that factor will be in the separation of populations. Factor analysis of morphometric characteristics led to the selection of 10 factors with eigenvalues of >1, explaining 73.83% of the variance of characteristics (Table 4).
|
%Cumulative |
of Variance% |
Eigen value |
Component |
|
10.767 |
10.767 |
2.907 |
1 |
|
20.380 |
9.613 |
2.596 |
2 |
|
29.376 |
8.996 |
2.429 |
3 |
|
38.298 |
8.922 |
2.409 |
4 |
|
45.437 |
7.139 |
1.928 |
5 |
|
52.074 |
6.637 |
1.792 |
6 |
|
58.522 |
6.448 |
1.741 |
7 |
|
64.422 |
5.900 |
1.593 |
8 |
|
70.087 |
5.664 |
1.529 |
9 |
|
73.831 |
3.744 |
1.011 |
10 |
Table 4 Eigenvalues, percentage of variance, and extracted factors of morphometric characteristics of male and female Hemiculter leucisculus
Pre-anal length and ventral anal length, ventral fin length, anal fin depth, caudal peduncle length and post-anal length, and after-eye length had factor coefficients of >0.75 in the first to the fifth factors, respectively. No characteristics had a factor coefficient of >0.75 in the sixth and ninth factors. Eye diameter, head width, and standard length had factor coefficients of >0.75 in the seventh, eighth, and tenth factors, respectively (Figure 2).
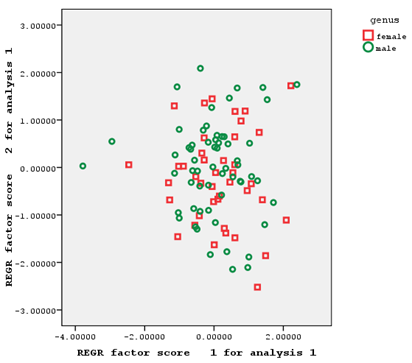
Figure 2 Individual distribution based on the first and second factors of morphometric characteristics of male and female Hemiculter leucisculus.
Factor analysis for meristic characteristics led to the selection of 3 factors with eigenvalues greater than 1, explaining 62.83% of the variance of characteristics (Table 5).
|
%Cumulative |
of Variance% |
Eigen value |
Component |
|
23.269 |
23.269 |
1.629 |
1 |
|
43.828 |
20.559 |
1.439 |
2 |
|
62.838 |
19.009 |
1.331 |
3 |
Table 5 Eigenvalues, percentage of variance, and extracted factors of meristic characteristics of male and female Hemiculter leucisculus
Brushed gill outer and brushed gill inner from the first factor, hard rays of the anal fin from the second factor, and the soft rays of the anal fin from the third factor had factor coefficients of >0.75 (Figure 3).
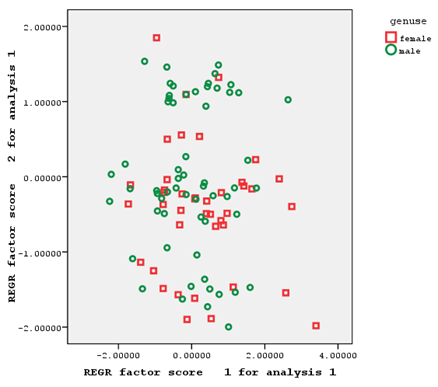
Figure 3 Individual distribution based on the first and second factors of meristic characteristics of male and female Hemiculter leucisculus
As the individual distribution based on the relationships of the first and second extracted factors of morphometric and meristic characteristics shows, the two sexes understudy had a significant overlap in terms of morphometric characteristics (with only a few number of samples separated from each other), making it impossible to separate male and female fish based on these characteristics (Figure 2). These two sexes also had a relatively high overlap in terms of meristic characteristics, which could not be a factor for the separation of the two sexes of fish (Figure 3).
There were high levels of intrapopulation variation based on the total coefficient of variation, which could be due to three factors of heterogeneous growth, the presence of more than one population in the region, and the presence of different phenotypic groups in the study area. Data standardization considerably reduces the effect of allometric growth, and it is possible to avoid the presence of different populations by sampling from a specific and limited area. Therefore, it is likely that most of the intrapopulation variation was due to different phenotypic groups in each area, associated with various environmental conditions or genetic differences.10 Morphometric measurements were mainly limited to body structures such as fins with limited ability to determine body shape as they tended to focus along the body axis. Samplings were only from the depth along the width and mainly in the head area.12
This study measured 27 morphometric and 10 meristic characteristics of male and female Hemiculter leucisculus fish. The mean coefficients of variation of morphometric characteristics of the female (18.144) and male (17.669) fish were close to each other, indicating almost equal environmental effects on morphometric characteristics of female and male fish populations in this river. Soule and Couzin-Roudy13 showed a negative correlation between the coefficient of variation and the heritability of morphological characteristics. In other words, environmental effects were more prominent than heritability in morphometric variation. Close means of the coefficient of variation in the two populations of male (10.714) and female (10.548) fish indicated a similar diversity of meristic characteristics in the two populations under study. However, the environmental factors did not affect meristic characteristics, and genetic factors were more influential. Winfield and Nelson14 stated that the variation of meristic characteristics did not depend on differences in environmental conditions, but primarily under the influence of hereditary and genetic factors.
The t-test results of 27 morphometric and 10 meristic characteristics of male and female sample fish showed no significant differences in 24 morphometric and all meristic characteristics of male and female fish (P>0.05) and significant differences in 3 morphometric characteristics, including head depth, dorsal fin depth, and pectoral fin base length (P≤0.05).
A comparison of factors extracted from multivariate analyses showed that the greater the range of variation of characteristics, the greater the number of extracted factors and eigenvalues of >1 in that group of characteristics.15 Factor analysis of morphometric characteristics led to 10 factors with eigenvalues of >1, explaining 73.831% of the variation in characteristics. Factor analysis of meristic characteristics led to 3 factors with eigenvalues of >1, explaining 62.838% of the variation in characteristics.
Mamuris et al.,16 stated that characteristics with a factor coefficient of >0.75 could separate populations. The first and second factors were used concerning the distributed clouds obtained by multivariate analyses because they had the highest eigenvalues, variance, and variability of characteristics.14 The distribution of individuals based on the relationships of the first and second extracted factors shows that the two sexes under study had a good overlap in terms of morphometric and meristic characteristics. Hence, it is not possible to separate the male and female fish based on these characteristics.
None.
The authors declare that there are no conflicts of interest.

©2023 Gorjian. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.