Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 2
1Department of Fisheries Technology, Bangladesh Agricultural University, Bangladesh
2WorldFish, Bangladesh and South Asia Office, Bangladesh
3Department of Fishing and Post-Harvest Technology, Chattogram Veterinary and Animal Sciences University, Bangladesh
Correspondence: MS Reza, Department of Fisheries Technology, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
Received: June 12, 2024 | Published: June 28, 2024
Citation: Osman MH, Islam SR, Uddin MN, et al. Microbiological analysis strategies for detecting pathogens and assessing biosecurity practices in outdoor earthen shrimp farms. J Aquac Mar Biol. 2024;13(3):64-69. DOI: 10.15406/jamb.2024.13.00398
The need for rapid and reliable on-farm bacteriological test methods to detect pathogens and assess the success of biosecurity practices in fish and shrimp farms has been increasing. This study compared on-farm and in-laboratory (after refrigerated transportation) analyses for black tiger shrimp (Penaeus monodon) cultured in two outdoor earthen ponds in low-resource settings on the southeastern coast of Bangladesh, using basic bacteriological facilities. Water quality parameters, including temperature, dissolved oxygen (DO), pH and salinity were determined at monthly intervals during summer (April to June). Water temperature in two farms ranged from 26.3°C to 29.3°C (27.7±1.6°C in Farm-1 and 27.4±0.9°C in Farm 2), within suitable limits for shrimp culture. DO levels varied from 5.1±0.2 to 5.4±0.5 mg/l, pH from 7.1±0.2 to 7.1±0.6, and salinity from 14.7±1.2 to 15.7±0.6 ppt, all within optimal ranges for shrimp farming. Bacterial loads showed slight variations between sampling periods. On-farm analyses consistently showed lower bacterial loads in shrimp samples compared to in-laboratory analyses with bacterial counts increasing over transportation time. Water temperature significantly influenced bacterial load as reflected by different sampling periods. The total bacterial load in farm water, sediment and shrimp samples showed notable differences between on-farm and in-laboratory analyses, reflecting the impact of environmental conditions, handling practices and time delays. It is concluded that on-farm microbiological analysis strategy is an effective method for detecting pathogens and assessing biosecurity practices in shrimp farms cultured in outdoor earthen ponds.
Keywords: biosecurity, enteric bacteria, specific pathogen-free, black tiger shrimp, on-farm microbiological testing
DO, dissolved oxygen; WSSV, syndrome virus; YHV, yellow head virus; TSV, taura syndrome virus; IHHNV, infectious hypodermal and hematopoietic necrosis virus; IMNV, infectious myonecrosis virus; EMB, Eosin methylene blue; SS, Salmonella-Shigella
Black tiger shrimp (Penaeus monodon) is the second most important shrimp species in aquaculture industry, cultivated across tropical and subtropical regions throughout the world.1,2. Renowned for its large size, this species has become an important species with high market demand in the shrimp farming industry. The high market demand is driven by its size, taste, and texture, making it a premium product in international seafood markets. The farming of black tiger shrimp began in earnest in the 1970s and 1980s, primarily in China, Thailand, Vietnam, and the Philippines.3 By the late 1990s and early 2000s, production of black tiger shrimp peaked in these regions with countries like Bangladesh and India also becoming key producers. However, this period also marked the onset of significant challenges, including disease outbreaks such as White Spot Syndrome Virus (WSSV) and Yellow Head Virus (YHV) that devastated shrimp populations and resulted in substantial economic losses.3,4 In 2018, the world production of black tiger shrimp stood at 750,600 tons.50 While production is making a strong comeback in many Asian countries due to advances in hatchery and farming techniques, challenges persist in countries like Bangladesh where disease outbreaks have been identified as the biggest threat to the development of shrimp aquaculture in the country.6 Besides WSSV and YHV, other significant viruses such as Taura Syndrome Virus (TSV), Infectious Hypodermal and Hematopoietic Necrosis Virus (IHHNV) and Infectious Myonecrosis Virus (IMNV) have also been reported.7,8
In aquaculture system, microorganisms residing in aquatic environment influence the water quality and are closely associated with the fish physiology and diseases. The success and profitability of aquaculture depends on water quality which is greatly influenced by aquatic microorganism. Numerous investigations have been carried out on the microbiology of freshwater and marine environment in different parts of the world. In every microbial habitat the nutritional competition between organisms plays an important role in influencing the composition of micro flora.9 It is well known that fish take a large number of bacteria into their gut from water, sediment and food.9–12 Al-Harbi and Uddin13 suggested that the bacterial flora on fish reflects the aquatic environment. Usually, aquatic animal including fish takes a large number of bacteria through their food and drinking water which accumulate in their intestine. Some of them present in the intestine for a relatively long period but most of which are temporary residents because of incompatible physical and chemical conditions, lethal interactions between bacteria or immune responses in the gut.10 The bacterial contents of the water also effects the quality and safety of the fish and fish products produced in the aquaculture settings.14,15 Since aquaculture practice in Bangladesh mostly rely on primary production and aquatic bacteria plays an important role on the productivity of aquaculture and production of good raw materials, it is important to know about the quantitative and qualitative estimates of micro flora residing in the culture system. Such information is necessary to detect disease causing and foodborne pathogenic bacteria as well as assessing the success of biosecurity practices being implemented in the shrimp farms. Although relatively new concept in Bangladesh, biosecurity practices are followed in many aquaculture facilities. These practices are essential to prevent devastating disease outbreaks, such as WSSV and AHPND. Implementing stringent biosecurity measures ensures sustainable shrimp production, enhances export quality, protects livelihoods, and boosts the economic contribution of the shrimp aquaculture sector to the national economy.16,17
In Bangladesh, shrimp farming and related activities contribute significantly to the national economy. The main areas of contribution are export earnings and employment generation for on and off farm activities.18 Over 15 million people are involved in different activities of shrimp industry in Bangladesh.19 A vast area in the coastal zones of Khulna, Satkhira, Bagerhat and Cox’s Bazar has been converted into shrimp farms. Bangladesh has about 2.5 million ha of brackish water, of which 0.13 million ha is under shrimp culture.20 To address disease challenges in the shrimp farms, there is an increasing demand for specific pathogen-free (SPF) black tiger shrimp broodstock.21 As the success of SPF broodstock is a part of comprehensive biosecurity in shrimp farming, there is a need to maintain a healthy and disease-free environment with the fish farms. However, there has been limited research conducted on this aspect in Bangladesh. Consequently, there is a scarcity of information on the bacterial load of shrimp intestinal contents, their variation and biochemical characteristics. Studies on aquatic microbiology are, therefore, crucial for the management of SPF broodstock, shrimp health and water quality. This knowledge is vital not only for sustainable aquaculture but also for public health considerations. Considering these facts, the present work aims to investigate the total bacterial load of water, sediment and shrimp samples, and identify the presence of resident enteric bacteria using two approaches, viz., on-farm, and in-laboratory after refrigerated transportation analyses and compared the results for selecting best analytical method of black tiger shrimp ponds.
Study area
Two black tiger shrimp farms (Figure 1) from Ukhia upazila in Cox’s Bazar district, Bangladesh were selected for this study. Both farms were supplied with ground water as a major source and surrounded by paddy fields. Limited vegetation was present in the shallow areas with bottom mud of approximately 15‒25 cm. Farms were supplied with artificial feed twice a day.
Figure 1 Map showing location of the shrimp farm at Ukhia upazila in Cox’s Bazar district (Source: Banglapedia).
Measurement of water quality parameters
On each sampling day water temperature, salinity, dissolved oxygen (DO) and pH of water and sediment were measured using previously calibrated handheld devices. All measurements were done in triplicate.
Sample collection
Sampling was done thrice at 1-month interval (1st sampling in April, 2nd sampling in May and 3rd sampling in June) for bacteriological investigations of pond water, sediment and whole black tiger shrimp in each of the two farms (designated as farm 1 and 2). In each farm, 3 locations were randomly selected and water samples were collected by submerging clean, sterilized bottles (250 ml) 15‒20 cm below the water surface at 3 locations and mixed together. Sediment samples, on the other hand, were collected from same 3 locations employing an alcohol rinsed, air dried small PVC pipe where the sample was aseptically retransferred to wide mouth sterile glass bottles (250 ml) and mixed together. Finally, 3‒4 black tiger shrimp (15‒20 g) were collected from the 3 locations. Sampling was conducted in duplicates and a total of 6 samples were taken from each farm where one set of sample was subjected to immediate analysis at farm-site and rest was kept in ice box with sufficient crushed ice to be transferred to the laboratory within 12‒14 has described previously.9
Bacteriological analysis on farm-site
Immediately after collection, all samples were taken to a small confined area covered with card-boards inside the office room with assistance of the farm owner. For water samples, dilutions were made (10-1 to 10-5) with sterile physiological saline (0.85% w/v NaCl) and aliquots of 0.1 ml of the serial dilutions were pipetted out and transferred aseptically to the sterile agar plates for total aerobic heterotrophic plate count by raising the upper lids sufficient enough to admit the tip of the pipette. The samples pipetted were spread by L- shaped glass rods throughout the surface of the media until the samples were dried out. Plates were then wrapped by aluminum foil and kept in room temperature before being transferred to the Laboratory of Microbiology, Department of Fisheries Technology, Bangladesh Agricultural University, Mymensingh, Bangladesh within 12‒14 h. The plates were incubated at 30°C for another 24-48 h. Only the plates having 30 to 300 colonies were considered to calculate bacterial population numbers and expressed as colony-forming units per unit of sample. For each sediment sample, 5 g of sediment was mixed with sterile saline solution to prepare 200 ml of stock solution and was similarly diluted (10-1 to 10-9) and transferred to the agar plate. Shrimp samples, on the other hand, were first ground and homogenized and approximately 5g of each homogenate was put in a bottle containing sterile saline solution to prepare 200 ml of stock solution. One milliliter of each homogenate solution was serially diluted (10-1 to 10-8) and treated in the same way as the water samples.22
Bacteriological analysis of ice stored sample in laboratory
The ice stored samples transported to the laboratory (named as in-lab analysis) were similarly subjected to bacteriological analyses.
Isolation and testing of bacteria
For both on-site and in-laboratory analyses, bacterial isolates were recovered and subjected to Gram-staining. Presence of enteric bacteria was determined by using selective media EMB- agar and SS- agar. From each stock solution of water, sediment and shrimp, 0.1 ml samples were transferred into the selective media. Growth of bacterial colony in EMB (Eosin methylene blue) agar and SS (Salmonella-Shigella) agar indicate the presence of enteric bacteria. For percent composition, the bacterial colonies were divided into different types according to the colony characteristics of shape, size, elevation, structure, surface, edge, color and opacity, and the number of colonies of each recognizable type was counted as described previously.23 Three to five representatives of each colony type were then streaked on agar plates repeatedly until pure cultures were obtained. The streaked agar plates were incubated at 30°C for two days. Discrete colonies from the streaked agar plates were transferred to agar slants.
Statistical analysis
The data obtained in the experiment were recorded and preserved in computer. The data obtained in the experiment were analyzed by using SPSS version 20.0 (SPSS Inc., Chicago, USA). Significant differences were determined among treatments at 5 % level (p < 0.05).
Physico-chemical parameters of water from shrimp farms
The average water quality parameters taken from the two culture farms are shown in Table 1. According to the sampling time, it was from early to late summer where water temperature was found to be decreasing gradually from 1st sampling (April) to 2nd sampling (May) and 3rd sampling (June). It is, therefore, has significant effect on physico-chemical parameters of farm water which is a very important aspect of shrimp farming. Although some water quality parameters varied between the 2 farms, water temperature during the sampling period ranged from 27.4 ± 0.9 to 27.7 ± 1.6°C while a significantly higher temperature was recorded for ambient air (30.6±1.9°C for farm 1 and 31.1±2.6°C for farm 2) (Table 1). Pond water pH was slightly alkaline (7.1 ± 0.2 to 7.2±0.6) while sediment was found to be of acidic nature (3.7 ± 0.5 to 3.9 ± 0.8). It was found that both farms had saline water with values ranging from 14.7±1.2 to 15.7±0.6 ppt while the DO varied from 5.1±0.2 to 5.4±0.5 ppm.
|
Farm No. |
Temperature (°C) |
pH |
Salinity (ppt) |
DO (mg/l) |
||
|
Water |
Air |
Water |
Sediment |
|||
|
1 |
27.7±1.6a |
30.6±1.9b |
7.1±0.6 |
3.9±0.8 |
14.7±1.2 |
5.4±0.5 |
|
2 |
27.4±0.9a |
31.1±2.6b |
7.1±0.2 |
3.7±0.5 |
15.7±0.6 |
4.9±0.2 |
Table 1 Physico-chemical parameters of water, air and sediment collected from two black tiger shrimp farms at Ukhia upazila in Cox’s Bazar district over three month’s period from April to June
*Values given are in mean±SD (n = 3).
**Values in a row with different superscript letters are significantly different (p < 0.05).
Quantitative estimation of bacterial flora
In this study, quantitative analyses of bacteria were conducted using standard plate count technique where we compared bacteriological estimates of on-farm (Figure 2) and laboratory testing (Figure 3). It was observed that during the period of study, on-farm microbiological testing showed bacterial loads in water, sediment and shrimp from farm 1 ranged from 5.83×103 to 4.11×104 cfu/ml, 1.02×108 to 5.96×108 cfu/g and 3.47×106 to 8.9×105 cfu/g during 1st sampling (April), 4.61×104 to 1.19×105 cfu/ml, 2.12×108 to 1.23×109 cfu/g and 1.25×106 to 1.34×107 cfu/g during 2nd sampling (May) and 2.08×104 to 3.01×105 cfu/ml, 6.19×107 to 4.15×109 cfu/g and 5.31×106 to 1.94×107 cfu/g during 3rd sampling (June) respectively. The same sample when tested in the laboratory after refrigerated transportation, bacterial load increased with values in farm water, sediment and shrimp ranging from 1.42×104 to 3.49×105 cfu/ml, 8.18×108 to 1.0×109 cfu/g and 5.48×106 to 2.53×107 cfu/g during 1st sampling, 2.01×105 to 3.37×105 cfu/ml, 6.01×108 to 3.03×109 cfu/g and 2.32×107 to 5.18×107 cfu/g during 2nd sampling and 6.02×105 to 1.22×106 cfu/ml, 6.25×109 to 9.94×109 cfu/g and 8.24×107 to 1.02×108 cfu/g during 3rd sampling respectively. As for the farm 2, we obtained more or less similar results for on-farm testing where bacterial loads were slightly higher than the previous farm where bacterial loads in water, sediment and shrimp ranged from 1.05×104 to 3.13×104 cfu/ml, 1.28×108 to 7.02×108 cfu/g and 1.88×106 to 4.38×106 cfu/g during 1st sampling, 2.62×104 to 1.42×105 cfu/ml, 3.26×108 to 1.52×109 cfu/g and 6.23×106 to 8.25×106 cfu/g during 2nd sampling and 5.37×104 to 2.32×105 cfu/ml, 2.41×108 to 4.63×109 cfu/g and 5.13×106 to 3.14×107 cfu/g during 3rd sampling respectively. Bacterial loads also increased in laboratory testing where the values for water, sediment and shrimp were 8.12×104 to 1.56×105 cfu/ml, 2.49×108 to 1.39×109 cfu/g and 1.03×106 to 1.55×107 cfu/g during 1st sampling, 3.52×104 to 6.10×105 cfu/ml, 7.02×108 to 1.97×109 cfu/g and 6.18×106 to 2.02×107 cfu/g during 2nd sampling and 5.27×105 to 1.44×106 cfu/ml, 6.35×109 to 9.15×109 cfu/g and 1.48×107 to 1.13×108 cfu/g during 3rd sampling respectively.
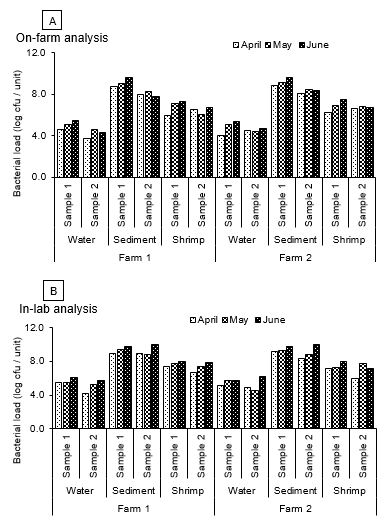
Figure 2 Bacterial load in water, sediment and shrimp samples for on-farm (A) and in-lab (B) analyses obtained from two black tiger shrimp farms at Ukhia upazila in Cox’s Bazar district. Samples were analyzed in duplicate (n = 2, namely sample 1 and 2).
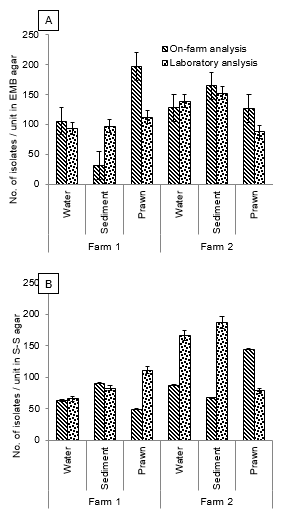
Figure 3 Enteric bacteria recovered from water, sediment and shrimp samples collected from two black tiger shrimp farms at Ukhia upazila, Cox’s Bazar district. A, isolates recovered from EMB-agar; B, those recovered from SS-agar (B).
Qualitative estimation of bacterial flora
We initially employed gram staining to assess bacterial populations, distinguishing species by their cell wall properties like peptidoglycan. Across the three different samplings (Table 2), farm 1 averaged 6 isolates each of water, sediment and shrimp comprising 35%, 32% and 40% G+ve respectively, with 65%, 68% and 60% G-ve bacteria. In farm 2, the counts were 6, 7 and 7 respectively, with 35%, 33% and 29% G+ve and 65%, 67% and 71% G–ve bacteria respectively. Salinity (14.7±1.2 to 15.7±0.6 ppt) likely influenced the prevalence of G-ve bacteria.
|
Sample |
1st sampling (April) |
2nd sampling (May) |
3rd sampling (June) |
||||||
|
No. of isolates |
G+ve |
G-ve |
No. of isolates |
G+ve |
G-ve |
No. of isolates |
G+ve |
G-ve |
|
|
Farm 1 |
|||||||||
|
Water |
6 |
2 (33%) |
4 (67%) |
5 |
2 (40%) |
3 (60%) |
6 |
2 (33%) |
4 (67%) |
|
Sediment |
7 |
2 (28%) |
5 (72%) |
7 |
2 (28%) |
5 (72%) |
5 |
2 (40%) |
3 (60%) |
|
Shrimp |
7 |
3 (42%) |
4 (58%) |
6 |
2 (33%) |
4 (67%) |
7 |
3 (43%) |
4 (57%) |
|
Total |
20 |
7 (35%) |
13 (65%) |
18 |
6 (33%) |
12 (67%) |
18 |
7 (38%) |
11 (62%) |
|
Farm 2 |
|||||||||
|
Water |
6 |
2 (33%) |
4 (67%) |
6 |
2 (33%) |
4 (67%) |
5 |
2 (40%) |
3 (60%) |
|
Sediment |
7 |
3 (43%) |
4 (57%) |
7 |
2 (28%) |
5 (72%) |
7 |
2 (28%) |
5 (72%) |
|
Shrimp |
7 |
2 (28%) |
5 (72%) |
8 |
2 (25%) |
6 (75%) |
6 |
2 (33%) |
4 (67%) |
|
Total |
20 |
7 (35%) |
13 (65%) |
21 |
6 (28%) |
15 (72%) |
18 |
6 (33%) |
12 (67%) |
Table 2 Gram +ve and Gram –ve bacteria isolated from water, sediment and shrimp samples obtained from two black tiger shrimp farms at Ukhia upazila in Cox’s Bazar district
*Values in the parentheses indicate percentage of the total bacterial isolates.
Later, we employed screening of enteric bacteria using selective media EMB and SS agar in a low-resource setting. Enteric bacteria like E. coli and Salmonella spp. were detected under on-farm and in-laboratory testing approach. On-farm results for farm 1 showed EMB counts of 105.6±6.5 cfu/ml, 31.3±7.0 cfu/g and 197±14.5 cfu/g respectively and SS counts of 63.0±9.0 cfu/ml, 90.6±4.5 cfu/g and 49.0±2.0 cfu/g respectively (Fig. 3). Post-transportation laboratory test results for farm 1 were EMB counts 93.3±6.0 cfu/ml, 97.0±2.2 cfu/g and 112.3±7.5 cfu/g respectively, and SS counts 65.3±1.8 cfu/ml, 82.3±8.7 cfu/g and 111.0±1.7 cfu/g respectively. Farm 2 on-farm test results showed EMB counts of 127.6±17.1 cfu/ml, 164.6±17.0 cfu/g and 126.3±18.3 cfu/g respectively, SS counts of 87.0±5.8 cfu/ml, 67.3±6.1 cfu/g and 144.6±9.2 cfu/g respectively. Laboratory results for farm 2 were EMB counts of 138.6±5.7 cfu/ml, 152.0±7.0 cfu/g and 88.0±3.2 cfu/g respectively, SS counts of 166.0±10.2 cfu/ml, 187.6±33.2 cfu/g and 78.3±6.3 cfu/g respectively, reflecting potential increases due to the transportation delays (under iced condition).
There is growing demand to develop efficient on-farm methods for rapidly detecting disease causing and foodborne pathogens in fish and shrimp. This study utilized basic In the present study, bacteriological facilities to compare on-farm and in-laboratory bacteriological analyses results in low-resource settings focusing on black tiger shrimp (P. monodon) in northeast Bangladesh. Water quality parameters were monitored monthly where temperature ranged from 30.6±1.9°C for farm 1 and 31.1±2.6°C for farm 2. It was reported that temperature below 14°C or above 35°C is lethal, 29-31°C being optimal.24 So, it can be said that water temperature was suitable for shrimp culture in this area. Haque et al.25 reported that the water temperature from 27.5 to 30.5°C was suitable for shrimp culture while Maclean et al.26 found that water temperature ranging from 28.9 to 29.1°C was favorable for the culture of shrimp. Microbes can grow at wide range of temperature. The suitable temperature for the growth of bacteria is 25 to 35°C, which is likely similar to our results. DO levels ranged from 5.1±0.2 to 5.4±0.5 ppm, aligning closely with previous reports.27,28 pH values in the studied farms ranged from 7.1 ± 0.2 to 7.2±0.6, conducive to bacterial growth with neutral pH being optimal.29 In the studied farms, the water salinity ranged from 14.7±1.2 to 15.7±0.6 ppt, similar to findings by Dewan et al.30 and Nirod.31 These results indicate suitable conditions for black tiger shrimp farming in this region.
Bacterial load varied slightly from one sampling to another sampling period. In on-farm analysis, bacterial load in all shrimp samples were lower than that of in-laboratory analysis. The quantitative changes of bacterial load in different samples with various conditions showed a characteristic pattern of increase in number with time. According to the kind of aquatic habitat, the composition of the bacterial flora differs widely dependent not only on the water’s content of organic and inorganic material, its pH, temperature and turbidity, but also on the sources from where organisms can enter into the water. In every habitat the nutritional competition between organisms plays an important role and influences decisively the composition of the microflora. Increasing bacterial load might be associated with water temperature has been documented in the literature.32,33 Al-Harbi34 reported the bacterial load in pond sediments as 3.2±1.2×105 to 2.8±1.5×107 cfu/g. The bacterial flora on fish is a reflection of the aquatic environment35 and it affects the storage life and quality of the fishery products. The growth of aquatic microorganisms is affected by a great variety of physical and chemical factors which, in a multitude of ways, may also act with or against one another. They influence not only the size and composition of the microbial populations, but also the morphology and physiology of the individual bacteria. In this study, total viable count of bacteria in water of farm 1 and 2 ranged from 5.83×103 to 3.01×105 cfu/ml in on-farm analysis; 1.42×104 to 1.44×106 cfu/ml in laboratory analysis. In sediment it was ranged from 6.19×107 to 4.63×109 cfu/g in on-farm analysis; 2.49×108 to 9.94×109 cfu/g in lab analysis. In shrimp it was ranged from 8.9×105 to 1.94×107 cfu/g in on-farm analysis; 1.03×106 to 1.13×108 cfu/g in lab analysis. This difference between on-farm analysis and in-laboratory analyses occurred due to the change of freshness and time interval. In this case, temperature may another factor in decreasing or increasing bacterial loads in the farm water, sediment and shrimp. Al-Harbi and Uddin36 reported that total viable counts were 6.7±2.1×l03 to 2.7±l.l×l03 cfu/ml in pond water. Total viable counts of bacteria (measured as colony-forming units, cfu) were in the range of 5.6±0.8×l03 to 2.4±1.2×104 cfu/ml in pond water; 1.9±1.5×l05 to 9.3±l.l×l06 cfu/g in sediment; and 3.4±1.8×l06 to 5.8±0.4×107 cfu/g in the intestine.13 These results are in agreement with our findings. The organic matter influences the load and composition of microbial population. Sediment bacterial composition and load are greatly influenced by effluent characteristics. On the other hand, bacterial flora in fish is the reflection of aquatic environments.37 It affects the storage life and quality of fishery products. The overall results indicated that the two farms were somewhat different in their bacteriological condition during the study period.
The presence of enteric bacteria in the farm water, sediment and shrimp samples suggested that water was polluted with various sources. There were inputs for sources of rainfall run-off and human or other animal feces directly to the farm and enteric bacteria were found in every farm. This finding relates to those of González et al.38 where they mentioned that the composition and level of the microorganisms associated with the intestinal tract is related to the environment as well as to the food consumed by fish. The results found in this study are comparable and similar to the results of the studies that have been conducted in different countries. It was reported that lowest number of total coliform and fecal coliform were found in the semi- intensive management system while the highest number of total coliform and fecal coliform were enumerated in traditional or extensive management system.39,40 From the present investigation, it was assumed that the bacterial load and number of enteric bacteria were higher because of semi-intensive systems where management system was poor.
Bacterial load increased significantly in farm water, sediment and black tiger shrimp samples showed a significant increase in-laboratory analysis compared to on-farm analysis. The presence of enteric bacteria in these samples from black tiger shrimp farms indicates contamination, possibly from feces of birds or other external sources. To maintain biosecurity, water safety for shrimp culture, it is crucial to identify and eliminate these contamination sources. Further investigation into the bacteriological composition of these farms is necessary to effectively manage and improve their health, productivity and sustainability. Finally, we propose on-farm analysis of shrimp farm samples can efficiently and accurately detect pathogens and assess biosecurity practices in shrimp farms cultured in earthen ponds.
This study was supported by a research grant under the core research project “Impact of Aquaculture Drugs and Chemicals on Aquatic Ecology and Productivity” with Grant No. BFRI/ARP/10/3161(4) from Bangladesh Fisheries Research Institute, Mymensingh, Bangladesh.
None.
We declare no competing financial interests. We state that the funding organization had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.

©2024 Osman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.