Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 3
1IPMA- Portuguese Institute for the Ocean and Atmosphere, EPPO- Aquaculture Research Station, Portugal
2IPMA- Portuguese Institute for the Ocean and Atmosphere, Divisão de Aquacultura, Valorização e Bioprospeção, Portuguese Institute for the Ocean and Atmosphere, Portugal
Correspondence: João Araújo, IPMA, Instituto Português do Mar e da Atmosfera (IPMA), Estação Piloto de Piscicultura de Olhão (EPPO)/ IPMA, Av. do Parque Natural da Ria Formosa s/n, 8700-194, Olhão, Portugal
Received: November 27, 2023 | Published: December 13, 2023
Citation: Araújo J, Cardoso C, Candeias-Mendes A, et al. Larval development, survival and fatty acid profile of purple sea urchin (Paracentrotus lividus Lamarck, 1816 (Echinodermata: Parechinidae) fed with Phaeodactylum tricornutum diatom. J Aquac Mar Biol. 2023;12(3):297-303. DOI: 10.15406/jamb.2023.12.00387
In this work, the effect of this microalga on the larval rearing of sea urchin Paracentrotus lividus was evaluated, and its use alone (D3) was compared with the application of multi-species diets (D1- Skeletonema costatum + Rhodomonas spp.+ P. tricornutum; D2- S. costatum + Rhodomonas spp.). To evaluate the success of the three diets, the development and survival of larvae fed the three diets was analyzed over 18 days. It was found that larvae fed the D3 diet grew and developed significantly faster than the other two diets (D1 and D2), but survival was significantly lower. To better understand the relationship between the larvae and their food, the fatty acid profile of the larvae fed the three diets was analyzed. The fatty acid profile of the three microalgae used was also analyzed. A correlation between the constitution of the larvae and their food was found, and the biosynthesis of some polyunsaturated acids, such as 20:5ω3 (EPA), 20:4ω6 (ARA), and 16:4ω3, was also verified. Despite the high nutritional value of P. tricornutum, it was found that this alga has a high EPA/ARA ratio, a ratio that may reduce larval survival. However, the association of this microalgal species with other species may contribute to improve the overall success of larval rearing.
Keywords: aquaculture, echinoderm, nutrition, microalgae, fatty acid
Marine resources are subjected to widely different anthropogenic pressures, being some of them frankly undervalued. That is the case of sea urchins, which are marine invertebrates of the phylum Echinodermata living on the ocean floor and encompassing more than 800 species. Gonads constitute the edible portion of sea urchins, accounting for approximately 10% of their total weight, however this value can vary widely depending on the environment (time of the year, temperature, food type and availability).1 These gonads are mainly composed by moisture, protein, lipid, and carbohydrate.2 Known for their specific aroma and excellent taste, sea urchin gonads are expensive and a delicacy in many countries, especially China and Japan, but still largely unexploited in the West. Global demand for sea urchins has increased significantly since the early 1970s, peaking at 113,654 tonnes landed in 1995, and has declined slightly until 2013, There was then a small recovery in the following years.3,4 Declining populations of wild sea urchins in recent decades, along with increased demand for sea urchin products, have stimulated scientific and commercial interest in aquaculture, increasing the amount of research to improve sea urchin farming5–7 for commercial and conservation/restoration purposes.5,7–10 The complexity of the biotic connections during the transitions between the larval and post larval phases of the echinoderm's life cycle and the existence of several stages throughout their life cycle make echinoderm aquaculture a challenge. Larval rearing is the most problematic phase in sea urchin culture, and high mortality is observed. Feeding is one of the most important factors in larval rearing, and it is essential to choose a food that is nutritionally adequate, easy to apply, and has low impact on water quality. Microalgae are the staple food for sea urchin larvae, and the choice and combination of species is one of the keys to the success of the larval stage. The larval microalgal diet selection is critical for overcoming problems with development, larval plasticity, deformities, competency, metamorphosis, inhibited growth, and mortality.11,12 The search for the optimization of larval diets has been the subject of many scientific works, such as Ahmed et al.,12 Carboni et al.,13 Castilla-Gavillan et al.,14 Araujo et al.,15 and Araujo et al.,16 In general, fatty acids play an important role in metabolic health in marine invertebrates as they have an essential role in several cell processes, supplying energy or regulating gene expression.17 Polyunsaturated fatty acids (PUFAs) have been identified as essential components of the cell membranes, determining not only the nutritional value of sea urchins, but also providing for normal growth and development of the organisms.18 Sea urchin larvae have a fatty acid profile characterized by a dominance of polyunsaturated fatty acids, with a particularly high percentage of high unsaturated fatty acids, mainly due to the high content of ARA (Arachidonic acid) and EPA (Eicosapentaenoic acid).19 These characteristics have been related to the great ability to assimilate this class of fatty acids through food, or even to their synthesis through precursors, such as linoleic acid and linolenic acid.20
This work focuses on the use of the diatom Phaeodactylum tricornutum. This microalga has properties that make it appealing to both aquaculture and the biochemical sector. It is currently utilized in the feeding of marine organisms, such as bivalve mollusk larvae and spat, penaeid prawn larvae, and live food species such as rotifers,21,22 which are then used to rear the larvae of marine fin fish and crustaceans.23,24 One of the main advantages of this microalga is the ability to obtain large amounts of biomass and therefore an alga with great potential for biodiesel production.25 In nutritional terms, it is characterized by the high content of the fatty acid EPA, an essential fatty acid involved in the formation of cell membranes, with recognized value for human health.26
The objective of this work was to evaluate the exclusive use of the diatom Phaeodactylum tricornutum (D3) in feeding sea urchin (Paracentrotus lividus) larvae, compared to two diets with mixture of species: D1 (Rhodomonas spp., Skeletonema costatum, Phaeodactylum tricornutum) and D2 (Rhodomonas spp., Skeletonema costatum). The choice of the other two species was due to their use in previous studies,15 thus serving these diets as controls.
Spawning induction
Wild purple sea urchins Paracentrotus lividus were brought to EPPO (Aquaculture Research Center) after being collected from intertidal rocks along the southern Portuguese coast. Breeders’ diet consisted primarily of fresh Ulva sp. collected in the saltwater reservoir at the EPPO facility, which was supplied daily by the tide cycles of the Ria Formosa lagoon. Maize grains (Zea mays) were also supplied. Feed was provided ad libitum, whenever there was a shortage in the tank. The spawning induction was carried out by injecting 1 ml of 0.5M KCl into the coelom through the peristomial membrane. Fertilization was carried out at a ratio of 500 spermatozoa per 1 egg after determining the number of oocytes. Fertilization took place in 5-litre glass beakers filled with filtered, UV-disinfected water. The adoption of this ratio, which has previously proven successful in our work, was based on a median value between values previously utilized in similar studies.11,27 After 2 hours, the fertilization success rate (about 100%) was verified by observing the presence of a fertilization envelope Fertilized eggs were hatched in 220L cylindrical fiberglass tanks with mild aeration and low light exposure for roughly 48 hours, in a room with the temperature kept at 20ºC. Cultivation was carried out in filtered and UV-disinfected water.
Microalgae culture
Skeletonema costatum, Rhodomonas spp., and Phaeodactylum tricornutum inoculum were obtained from IPMA algae collection (Portugal). For seed cultures, 60L of F2 medium was incubated with 10L of dense laboratory culture. The 60L plastic bag photobioreactor was incubated at 20±0.5 ºC under ≈100 µmol photons m-2 s-1 for an 18/6h light/dark cycle with aeration. Biomass was collected between 7 to 10 days of cultivation during the stationary phase.
Larvae rearing experiment
The larval cultivation experiment was conducted in twelve 6L flat bottom flasks (three replicates for each treatment) with no water inflow and low aeration (enough to help the larvae movement on the water column). The room temperature was kept constant to keep the water at 19 °C (+-0.1). Approximately 17500 larvae were introduced in each 6L flat bottom flask (replicate) filled entirely with filtered and UV-disinfected water, resulting in an approximate initial density of approximately 2.9 larvae per ml. Daily partial water renewals of 30% of the total water volume were achieved utilizing a siphon with reverse filtration via a 30m filter. The physical impact on the larvae was minimal due to the vast surface area of the filter and the low flow of the siphon. During the larval rearing experiment, three diets were tested: a mixed diet (approximately the same number of cells/l each species) of Rhodomonas spp., S. costatum, Phaeodactylum tricornutum (Diet 1- Rm+Sc+Pt), and a mixed diet of Rhodomonas spp., S. costatum, (Diet 2- Rm + Sc), and a single species diet of P. tricornutum. (Diet 3- Pt). The daily amount of microalgae cells was calculated according to the larval stage of development, following the method described by Carboni et al.13
Sampling
At days 5, 8, 15, and 18, larval counts were performed to determine the survival rate. Biometric sampling was also performed on these days. Measurements of the following parameters were performed: total length (TL), post-oral arm length (PL), body length (BL), stomach diameter (SD), width, (W), and rudiment length (RL). The term "rudiment" typically refers to the initial stages of development of certain structures or organs that will later become more developed and specialized in the adult sea urchin (Figure 1). Samples of 30 mL were taken from each 6L replicate flasks after gentle stirring to homogenize the culture water and larvae were counted using a 10mL counting chamber, under a Nikon SMZ 1000® stereomicroscope equipped with a Nikon DS-Fi2® camera. Image analyses were made using a Nikon DS-L3® Viewer. The survival rate was assessed using the following equation:
T=time of observation
T0=beginning of the trial
To characterize the development of the larvae, the transition between the different larval stages was periodically observed and the percentage of larvae in each stage of development was estimated. Three stages of development were considered: 4 arms, 6 arms and 8 arms. Identification was made through microscopic observation.
Fatty acid profile analysis
Biochemical analyses were performed to determine the fatty acid profile of the microalgae used in this work (Rhodomonas spp., S. costatum, P. tricornutum) as well as the sea urchin larvae from each of the treatments. To obtain microalgae samples for this analysis, three 2 L replicates of microalgae were centrifuged for 10 minutes at 8000 × g using a Z383K centrifuge (HERMLE Labortechnik GmbH C, Wehingen, Germany). The resulting biomass was cryopreserved at -80 ºC until the moment of analysis. The samples were taken from the bags used to feed the larvae, with the microalgae still in the stationary phase. To obtain larval samples, filtration was performed on the last day of the assay (18 days after hatching- DAH) to collect all surviving larvae for each treatment. Due to the low biomass obtained with the filtration, it was not possible to obtain replicates, and only one sample was analyzed for each treatment.
Fatty acid methyl esters (FAME’s) of total lipids were prepared by acid-catalyzed transesterification using Lepage & Roy method,28 modified by Cohen, Vonshak, Richmond.29 A 0.3g freeze-dried sample was saponified and transesterified with methanol:acetyl chloride solution (19:1) and the methyl esters were extracted into n-heptane. The internal standard used was saturated fatty acid heneicosanoic acid (21:0). FAME were separated and quantified using a Scion 456-GC gas chromatograph (West Lothian, UK), equipped with a capillary column DB-WAX (Agilent Technologies, Santa Clara, CA, USA) (film thickness, 0.25 mm), 30 m × 0.25 mm i.d. The separation of the FAME was carried out with helium as the carrier gas, using a temperature program for the column starting at 180 ºC and increasing to 200ºC at 4 ºC/min, holding for 10 min at 200ºC, heating to 210ºC at the same rate, and holding at this temperature for 14.5 min. Peak identification and quantification were done by comparing the relative retention times between each FAME from sample tissue and the reference standards for the most common FAMEs (Sigma Kit no. 189-19, ST Louis MO, USA). Fatty acids are expressed in mg/g.
Statistical analysis
All results obtained were statistically compared to determine the existence of potential differences between treatments (One-Way ANOVA) when equal variances between treatments, or Mann-Whitney Rank Sum Test, when unequal variances between treatments) with a 95% confidence interval, that is, p <0.05. The Shapiro-Wilk test was used to check the normality of the samples. The Tukey test was performed for determining the difference among all treatments. All analyses were carried out with Sigmaplot® software.
Survival and larval development
Larval survival was similar for the three treatments on days 5, 8 and 15 DAH, reaching a minimum mean value of 58.9 (±14.0) % for larvae fed exclusively on P. tricornutum. Between day 15 and the end of the trial (18 DAH), there was a marked drop in survival for the diet 3 (Pt) treatment, lowering the value to 25.7 (±12.1) %. Survival of larvae fed diet 1 (Rm+Sk+Pt) and diet 2 (Rm+Sk) remained relatively stable, being significantly higher than diet 3 (Pt) at the end of the trial (Figure 1).

Figure 1 Survival rate of larvae of Paracentrotus lividus at the different development stages at ages 5, 8, 15, and 18 DAH (days after hatching). Three different diets: D1 (Rhodomonas spp., Skeletonema costatum, and Phaeodactylum tricornutum D2 (Rhodomonas spp., Skeletonema costatum), D3 Phaeodactylum tricornutum). Values correspond to the mean value of the replicates of each treatment in percentage, with the corresponding error bar to represent the standard deviation. Values with different letters show significant differences (P<0.05).
The proportion of larvae at each stage of development (4, 6 and 8 arms) is shown in Figure 2. It was observed that larvae fed exclusively on P. tricornutum showed greater development at the end of the 8 DAH. At this point in the trial, 67% of the larvae fed diet 3 (Pt) were already in the 6-arm larval stage, while those fed the other two diets (Rm+Sk+Pt and Rm+Sk) were all in the early 4-arm stage. In the following sampling points (15 and 18 DAH) a strong presence of larvae in the 6-arms state was already observed in the three treatments, being the predominant state. At the end of the trial, the treatments that included the microalgae P. tricornutum had a higher percentage of larvae in the final stage of development (8-arms), reaching a maximum value of 57% on diet 3 (Pt).
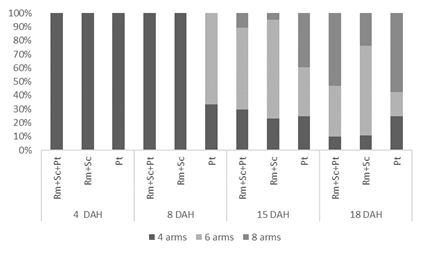
Figure 2 Percentage of larvae of Paracentrotus lividus at the different development stages (4, 6 and 8 abrms) at ages 5, 8, 15, and 18 DAH (days after hatching). Three different diets: D1 (Rhodomonas spp., Skeletonema costatum, and Phaeodactylum tricornutum), D2 (Rhodomonas spp., Skeletonema costatum), D3 Phaeodactylum tricornutum).
The biometric measurements recorded in each sampling are described in the Table 1. On days 15 and 18 DAH’s mean total length (TL) of larvae was significantly higher on diet 3 (Pt). These larvae also had higher values regarding stomach diameter (SD), larval width (W), and rudiment length (RL). In general, it was found that larvae fed diets 1 (Rm+Sc+Pt) and 2 (Rm+Sc) had similar biometric dimensions on days 15 and 18 DAH. The greater development of larvae fed exclusively with P. tricornotum is also evident from the index of the ratio of rudiment length to stomach diameter, a parameter that gives us information about the state of competence of the larva to initiate fixation and metamorphosis to the benthic phase (Figure 3). Larvae on diet 3 (Pt) on day 18 DAH were significantly better prepared for phase transition.
|
|
Diet |
5 |
8 |
15 |
18 |
|
Total length (TL) |
Rm+Sc +Pt |
716 ± 49 |
746 ± 50 a b |
769 ± 108 a |
810 ± 100 a |
|
|
Rm+Sc |
698 ± 45 |
692 ± 55 a |
773 ± 77 a |
901 ± 150 a |
|
|
Pt |
718 ± 48 |
812 ± 114 b |
861 ± 167 b |
1087 ± 132 b |
|
Post-oral lenght (POL) |
Rm+Sc +Pt |
351 ± 60 |
385 ± 40 |
386 ± 62 a |
388 ± 89 a |
|
|
Rm+Sc |
380 ± 60 |
378 ± 71 |
382 ± 59 a |
469 ±105 b |
|
|
Pt |
363 ± 45 |
422 ± 74 |
435 ± 106 b |
512 ± 97 b |
|
Body lenght (BL) |
Rm+Sc +Pt |
405 ± 25 |
404 ± 42 a b |
415 ± 62 a |
486 ± 58 a |
|
|
Rm+Sc |
120 ± 21 |
384 ± 44 a |
420 ± 52 b |
512 ± 94 a |
|
|
Pt |
386 ± 69 |
443 ± 51 b |
453 ± 89 a |
616 ± 83 b |
|
Stomach diameter (SD) |
Rm+Sc +Pt |
120 ± 14 |
99 ± 17 a b |
106 ± 29 a |
130 ± 23 a |
|
|
Rm+Sc |
135 ± 4 |
96 ± 17 a |
101 ± 32 a |
112 ± 32 a |
|
|
Pt |
122 ± 4 |
125 ± 41 b |
135 ± 59 b |
283 ± 113 b |
|
Width (W) |
Rm+Sc +Pt |
|
280 ± 58 a b |
287 ± 71 a |
386 ± 74 a |
|
|
Rm+Sc |
|
254 ± 54 a |
291 ± 48 a |
336 ± 58 a |
|
|
Pt |
|
350 ± 76 b |
266 ± 59 b |
563 ± 161 b |
|
Rudiment lenght (RL) |
Rm+Sc +Pt |
|
|
151 ± 38 |
87 ± 17 a |
|
|
Rm+Sc |
|
|
171 ± 23 |
73 ± 19 a |
|
|
Pt |
|
|
271 |
154 ± 84 b |
Table 1 Biometric measurement of larvae of Paracentrotus lividus at the different development stages at ages 5, 8, 15, and 18 DAH (days after hatching). Three different diets: D1 (Rhodomonas spp., Skeletonema costatum, and Phaeodactylum tricornutum), D2 (Rhodomonas spp., Skeletonema costatum), D3 (Phaeodactylum tricornutum). Values correspond to the mean value of the replicates of each treatment in microns. Values with different letters show significant differences (ANOVA, P<0.001)

Figure 3 Ratio of rudiment diameters to stomach diameters of Paracentrotus lividus at the different development stages at ages 5, 8, 15, and 18 DAH (days after hatching). Three different diets: D1 (Rhodomonas spp., Skeletonema costatum and Phaeodactylum tricornutum), D2 (Rhodomonas spp., Skeletonema costatum), D3 Phaeodactylum tricornutum).
Fatty acid analysis
Analyses were carried out to characterize the fatty acid profile of the microalgae used in the experiment (Rhodomonas spp., S. costatum, and P. tricornutum) as well as the larvae of each treatment (diets 1, 2, and 3), with the absolute values (mg/g) represented in the Table 2.
|
Microalgae |
Larvae |
|||||
|
Fatty Acid (mg/g) |
Diet 1 |
Diet 2 |
Diet 3 |
|||
|
|
Rhodomonas spp. |
Skeletonema costatum |
Phaeodactylum tricornutum |
Rm+Sc+Pt |
Rm+Sc |
Pt |
|
14:0 |
6.74 ± 0.30 |
15.30 ± 3.00 |
6.15 ± 0.19 |
0.68 |
0.64 |
0.68 |
|
16:0 |
12.02 ± 0.04 |
41.94 ± 7.22 |
29.25 ± 2.33 |
3.719 |
3.93 |
4.45 |
|
18:0 |
0.40 ± 0.02 |
0.79 ± 0.20 |
0.57 ± 0.07 |
1.23 |
1.53 |
1.65 |
|
Other SFA |
5.80 ± 0.30 |
3.94 ± 0.82 |
3.15 ± 0.11 |
1.49 |
1.41 |
0.86 |
|
Total SFA |
24.95 ± 0.64 |
61.89 ± 11.15 |
39.12 ± 2.61 |
7.12 |
7.51 |
7.64 |
|
16:1 (ω9+ω7) |
0.68 ± 0.01 |
40.37 ± 11.14 |
42.99 ± 3.04 |
1.44 |
0.31 |
2.85 |
|
18:1ω9 |
2.51 ± 0.18 |
9.55 ± 5.47 |
5.67 ± 0.66 |
0.60 |
0.35 |
0.48 |
|
18:1ω7 |
2.62 ± 0.03 |
0.89 ± 0.10 |
1.29 ± 0.95 |
1.45 |
0.93 |
4.78 |
|
20:1 (ω11+ω9+ω7) |
0.01 ± 0.02 |
0.85 ± 0.73 |
0 |
1.26 |
1.94 |
2.76 |
|
Other MUFA |
0.52 ± 0.04 |
0.96 ± 0.41 |
0.36 ± 0.09 |
0.77 |
0.63 |
1.34 |
|
Total MUFA |
6.33 ± 0.26 |
52.66 ± 5.36 |
49.00 ± 3.67 |
5.55 |
4.16 |
12.31 |
|
16:4ω3 |
0.19 ± 0.01 |
0.1 ± 0.04 |
0.49 ± 0.12 |
1.94 |
3.17 |
6.02 |
|
18:2ω6 |
13.31 ± 0.52 |
1.49 ± 1.37 |
0.97 ± 0.12 |
1.07 |
1.28 |
0.31 |
|
18:3ω3 |
24.71 ± 1.02 |
2.30 ± 1.36 |
0.32 ± 0.05 |
0.52 |
0.57 |
0.09 |
|
20:4ω6 ARA |
1.50 ± 0.03 |
1.16 ± 0.34 |
0.13 ± 0.03 |
3.00 |
5.78 |
2.69 |
|
20:5ω3 EPA |
10.21 ± 0.20 |
13.32 ± 5.15 |
14.81 ± 0.61 |
4.45 |
4.09 |
11.29 |
|
22:6ω3 DHA |
8.41± 0.04 |
8.15 ± 3.34 |
1.21 ± 0.06 |
1.72 |
2.97 |
0.83 |
|
Other PUFA |
22.03 ± 0.95 |
16.40 ± 2.18 |
4.79 ± 0.18 |
1.02 |
1.44 |
1.97 |
|
Total PUFA |
80.18 ± 2.76 |
43.08 ± 2.04 |
22.23 ± 1.03 |
16.66 |
23.86 |
25.34 |
|
Total PUFA (ω3) |
64.30 ± 2.01 |
35.14 ± 2.82 |
18.43 ± 0.84 |
10.20 |
12.27 |
19.85 |
|
Total PUFA (ω6) |
15.82 ± 0.75 |
3.62 ± 0.85 |
1.13 ± 0.17 |
5.87 |
11.19 |
4.04 |
|
EPA+DHA |
18.62 ± 0.25 |
21.47 ± 1.95 |
16.02 ± 0.66 |
6.18 |
7.07 |
12.12 |
|
Ratio ω3/ω6 |
4.06 |
10.16 |
16.52 |
1.74 |
1.10 |
4.92 |
|
Ratio DHA/EPA |
0.82 |
0.61 |
0.07 |
0.39 |
0.73 |
0.07 |
|
Ratio EPA/ARA |
6.80 |
11.48 |
113.92 |
1.48 |
0.71 |
4.20 |
|
Total FA |
111.5 ± 1.75 |
155.94 ± 14.35 |
110.35 ± 6.88 |
29.3 |
35.5 |
45.3 |
Table 2 Fatty acid profile of the three microalgae (Rhodomonas spp., Skeletonema costatum, and Phaeodactylum tricornutum) and sea-urchins larvae (Paracentrotus lividus) fed the three different diets: D1 (Rhodomonas. spp.., S. costatum and P. tricornutum), D2 (Rhodomonas spp., S. costatum), and D3 Phaeodactylum tricornutum), collected at the age of 18 DAH. Absolute values (mg/g)
Based on the results of this work, we can conclude that the best strategy for feeding sea urchin larvae is to provide a mixture of different microalgae that guarantees a balanced supply of essential fatty acids for the development and survival of the larvae. The exclusive use of the diatom Phaeodactylum tricornotum was considered insufficient for effective feeding, however, together with other microalgae, this microalga contributes to good development during the larval stage.
Fatty acid profile
We begin the discussion with an examination of the fatty acid profile for the information gathered to be used later in the examination of the larval culture test in the issue. The examination of these results is limited due to the inability to obtain replicates for each treatment. The surviving larvae of each replicate were filtered at the end of the test, but there was insufficient biomass for the study. As a result, pool samples of each treatment were taken to achieve the required biomass. Fatty acids play a crucial role in the physiology and reproduction of marine organisms. Omega-3 fatty acids, specifically EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) are essential for the development of the nervous and immune system in marine organisms.30,31 It has been determined that echinoderms in general are a superior source of fatty acids for human nutrition. Unusual fatty acids found in its constitution come from its mode of feeding and capacity for biosynthesis.32 One of the objectives of this work was to try to understand the importance that feed has on the fatty acid profile of sea urchin larvae and relate it to their quality. The first observation is that the total fatty acid content of the larvae, in absolute values, is much lower than in the provided feed, making it possible to realize that the supply of microalgae richer in fatty acids may not fully translate into larvae with higher content of fatty acids. Nonetheless, there is an influence of the diet on the fatty acid composition of these larvae, which agrees with previous findings in sea urchins in general.12 The microalgae Skeletonema costatum was the species that presented the highest content of total fatty acids, however larvae fed on Phaeodactylum tricornutum had higher total FA.
Microalgae have a higher total content of saturated fatty acids compared to larvae, with stearic acid (18:0), being the exception to the rule. For the three treatments, it was observed that the larvae had more 18:0 than the feed, which could show that the larvae incorporate this FA into their body composition. This result agrees with previous work by Liu et al.,20 which recorded a higher content of this FA in larvae relative to the feed. This may be associated with an accumulation of this fatty acid during the development of the larvae or of embryonic origin. According to Gago et al.19 eggs released by wild P. lividus adults have a 3.44% 18:0 content, and the larvae originated from these eggs have a 4.35% content. In the current study, the larvae had content between 1.23 and 1.65%, depending on the treatment; very similar values were reported in the referred works.19,20 The analysis of these works seems to suggest that the origin of this fatty acid is essentially embryonic, with a slight accumulation in the composition of the larvae during the larval stage.
Similarly to SFA, microalgae have a much higher total content of monounsaturated fatty acids (MUFA) than larvae, with this difference being relatively small when comparing the profile of the larvae with the microalga Rhodomonas spp. The high content of MUFA in the microalgae S. costatum and P. tricornutum is mainly due to 16:1 (palmitoleic acid), a fatty acid that is a component of the phospholipid fraction of cell membranes. However, this acid is present in only small amounts in the larvae of the three treatments. Within the MUFA class, it was also found that larvae have a higher content of 20:1 compared to the microalgae used for feeding, whose observed content was very low. Gago et al.19 found that P. lividus eggs had relatively high levels of 20:1, being possible that the origin of this fatty acid in the larvae is possibly embryonic.
The total absolute content of polyunsaturated fatty acids in microalgae was higher than in larvae for diets 1 and 2 (due to the presence of Rhodomonas spp.), a situation that did not occur in larvae fed exclusively with P. tricornutum, where this value was higher in the larvae. Rhodomonas spp. was the microalgae with the highest PUFA abundance, with the fatty acid 18:3ω3, also known as alpha-linolenic acid (ALA), contributing the most to this abundance. This microalga is naturally rich in this fatty acid and can represent 25% of total fatty acids.33 For the other two microalgae used and analyzed (S. costatum and P. tricornutum), the most abundant PUFA was 20:5ω3, also known as eicosapentaenoic acid (EPA), an essential fatty acid involved in the formation of cell membranes. PUFAs were the most abundant class of fatty acids in sea urchin larvae for the three treatments used (diets 1, 2, and 3), with EPA being one of the most abundant fatty acids. Comparing the fatty acid profile of the larvae and their feed, it was found a significant retention of the fatty acids 16:4ω3 and 20:4ω6 in the composition of larvae fed with the three diets. The fatty acid 16:4ω3, also known as hexadecatetraenoic acid, was found in the larvae in absolute quantities higher than the algae used in their diet. This abundance was higher in larvae fed with diets 2 (Rm+Sc) and 3 (Pt), and curiously lower in larvae fed with diet 1, which included the three algae used. In the consulted literature, the presence and importance of this fatty acid in the composition of sea urchin larvae13,18–20 was not confirmed. Its presence in the analyzed larvae may be due to biosynthesis through other precursor fatty acids. The 20:4ω6 fatty acid, also known as arachidonic acid (ARA), is an essential component of the biological cell membrane, providing it with the fluidity and flexibility required for all cells to operate.34 Like the previous one, this fatty acid was present in the sea urchin larvae at higher levels than in the microalgae used in the diet, this difference being particularly relevant in larvae fed with diet 3 (P. tricornutum). While this microalga species presents almost vestigial contents of 20:4ω6, it reached in the larvae an amount about 20 times higher. In the work of Gago et al.,19 it was found that this fatty acid appears in significant quantities in sea urchin eggs, increasing this concentration progressively after hatching and formation of the 4-armed larvae. The work carried out by Carboni et al.13 showed a decrease in the ARA content after hatching, followed by an increase until the end of larval development. Arachidonic acid is an essential fatty acid, which can be formed through biosynthesis using linoleic acid, or 18:2ω6, as the main precursor fatty acid.34 In a similar work, it is suggested that sea urchins are able to biosynthesize ARA using also other ω 6 precursors, such as 18:3ω6 and 20:3ω6.13,35
It should also be emphasized the sensitivity of the larval fatty acid profiles to the microalgal specific fatty acid composition in the cases of the ω3/ω6 and the EPA/DHA ratios. On the other hand, the response of the profile of the larvae fed diet 2 to the large amount of 16:0 and 16:1 (ω9 + ω7) in S. costatum was very attenuated or even absent. Furthermore, the high content of 18:3 ω3 in Rhodomonas spp .was not reflected in the fatty acid profile of larvae fed diet 1, but also failed to substantially elevate the levels of EPA and DHA, which can be synthesized from 18:3 ω3. This latter result deserves special attention. In fact, several authors, such as Kabeya et al.,18 have demonstrated that P. lividus has desaturases that account for all the desaturation reactions required for the biosynthesis of the physiological essential EPA and ARA through the “Δ8 pathway”. However, the EPA’s lack of accumulation contrasts with that of ARA. This may be a case of competition between ω6 and ω3 substrates for the action of the enzymes and a consequence of the relative abundance of ω3 PUFA and, in particular, EPA in the dietary microalgae (see their ω3/ω6 ratios), thus favoring a “redirection” of the desaturases to ω6 substrates.
Survival
The survival of the larvae in the first days of the test was very similar, with no significant differences being observed up to 15 DAH. On the 18th day, the situation changed, with a drop in the survival of the larvae fed exclusively with the microalgae P. trichornutum (3 Pt diet). At the end of the trial, which corresponds to day 18 DAH, the estimated survival of larvae fed diet 3 (Pt) was 25.7%, for the other treatments it was 66.9% for diet 1 (S. costatum, R. marina, and P. trichornutum) and 61.7% for diet 2 (S. costatum and R. marina). In other similar studies carried out at our facilities with P. lividus , Araújo et al.15 and Araújo et al.16 obtained maximum final survivals of 75.8% for larvae fed with R. marina, S. costatum, and Emiliana huxleyi and 58% for larvae fed with S. costatum and R. marina. In the work of Carboni et al.8 the authors obtained a survival of approximately 45% for larvae fed on Dunaliella tertiolecta. In Castilla-Gavilan et al.14 the highest survival was in P. lividus larvae fed exclusively on Rhodomonas sp., reaching 25% after 15 days after hatching. Ahmed et al.12 maximum survival for the same age was 26.25% for larvae fed a mixture of three species: Chaetoceros calcitrans, Nannochloropsis oculata, and Tetraselmis suecica. Gomes et al.36 conducted a similar study with P. lividus and with the microalgae P. tricornutum and obtained a survival of about 25% at the age of 18 DAH when fed exclusively with this species. In the same work, they combined this microalga with Rhodomonas spp. However, survival was below that in the previous diet. Comparing our results with the cited works, survivals were relatively high, especially in larvae fed with the mixture of the three microalgae. The exclusive use of P. tricornutum does not seem to be a good option when the main objective is to obtain high levels of survival.
Growth and development
The availability and quality of feed as well as the temperature of the medium influence the growth and development of sea urchin larvae. Temperatures that are too low reduce growth and increase the rate of larval malformation. For the cultivation of sea urchin larvae at the EPPO facilities, a room with a stabilized temperature of 19-20 ºC is used, with no daily or seasonal fluctuations. According to Pétinay, Chataigner, and Basuyaux,37 the ideal temperature to ensure a good growth rate and promote larval development is between 18 and 21.5 ºC. Considering that all replicates were subjected to the same environmental conditions, the differences in development should be directly related to the quality of the feed. In this work, it was found that sea urchin larvae developed faster on a diet exclusive of P. tricornutum, particularly in the first week after hatching. This was seen both in biometry and in the progression of developmental stages. At the end of the trial, 18 days after hatching, larvae fed on P. tricornutum also had a higher rate of competence for metamorphosis and attachment. The quality of the fatty acid profile is one of the most important properties in choosing a good feed for sea urchin larvae. According to Liu et al.20 this choice should mainly consider the ratio between fatty acid pairs such as DHA/EPA and EPA/ARA, with the absolute content being secondary for adequate sea urchin larval development. Carboni et al.13 states that a good feed should have low DHA/EPA ratio value, and therefore a feed with high EPA content is favorable. For another sea urchin species (Dendraster excentricus), Schipiou et al.35 states the importance of the total content of PUFA (ω3) type fatty acids, also mentioning the advantage of high EPA feed over DHA. In this work, the advantage of a high EPA/ARA ratio is also emphasized.38 In the present work, the microalga species in focus (P. tricornutum) was found to have a low DHA/EPA ratio relative to the other microalgae, which may explain the greater development of larvae fed exclusively on this microalgae. However, the EPA/ARA ratio of P. tricornutum was found to be much higher than the other microalgae, which is primarily a disadvantage for the larvae. This unfavorable ratio could possibly be one of the reasons for the lower survival of larvae fed exclusively on these microalgae.
Based on the results of this work and other similar studies, we can conclude that the best strategy for feeding sea urchin larvae is to provide a mixture of different microalgae that guarantees a balanced supply of essential fatty acids for the development and survival of the larvae. The exclusive use of the diatom Phaeodactylum tricornotum was considered insufficient for effective feeding, however, together with other microalgae, this microalga contributes to good development during the larval stage.
This study had the support of the project WP9- Portuguese Blue Biobank under the Blue Economy Pact - Project Nº. C644915664-00000026
The authors have no relevant financial or non-financial interests to disclose.

©2023 Araújo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.