Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 1
Department of Business and Primary Industries, Australia
Correspondence: Sadiqul Awal, Department of Business and Primary Industries, Bachelor of Agriculture & Technology degree program (Aquaculture), Melbourne Polytechnic, Cnr Cooper St & Dalton Rd, Epping, Victoria 3076, Australia, Tel 61392691162
Received: November 24, 2015 | Published: February 19, 2016
Citation: McDowall S, Awal S, Christie A (2016) Investigation into the Potential use of Inland Saline Groundwater for the Production of Live Feeds for Commercial Aquaculture Purposes. J Aquac Mar Biol 4(1): 00071. DOI: 10.15406/jamb.2016.04.00071
Traditional agricultural methods and practices have rendered over 100 million hectares of land throughout the world, and over 5.7 million hectares in Australia, unsuitable for most forms of agriculture due to elevated salinity levels. Inland saline aquaculture is an adaptive approach to this environmental problem, and represents a potentially lucrative use for salt-affected land, with many economic, social and environmental benefits possible. Perhaps surprisingly, to date there has been relatively very little research conducted into the suitability (or otherwise) of inland saline aquaculture for the production of various species of microalgae and live feeds, which represent a crucial segment of the aquaculture industry.
The key aim of this investigation was to test the hypothesis that all saline waters (natural sea water, artificial sea water and inland ground saline water) would produce uniform growth amongst the live feeds tested. This study expanded on the live feed species being tested to include Artemia (Artemia salina) rotifers (Brachionus plicatilis) and copepods (Cyclop ssp). While there were significant differences in the performance of all the tested live feed species, Nannochloropsis oculata and Brachionus plicatilis showed better growth rates than those observed for natural seawater. It is hoped that these results can be used proactively by farmers seeking to diversify their crops to include the aquaculture of finfish in salt-affected areas of Australia and elsewhere.
Keywords: Live feed, Rotifer, Copepod, Artemia, Brachionus plicatilis, Cyclops spp, Nannochloropsis oculata, SGW, ASW, Neubauer haemocytometer, Dryland salinity
SGW, Saline Ground Water; ASW, Artificial Saline Water; PMSEIC, Prime Minister’s Science, Engineering and Innovation Council (of Australia); NLWRA ,National Land and Water Resources Audit; TDS, Total Dissolved Solids; CSIRO, Commonwealth Scientific and Industrial Research Organisation; Na+, Sodium; K+, Potassium, Ca2+, Calcium, Mg2+, Magnesium; SO42-, Sulphate Cl-, Chloride
The production of live feeds is a crucial segment of the global aquaculture industry. In effect, aquaculturists seek to develop a simplified food web, which utilises algae and zooplankton to feed larval fish. Algae, which form the base of the food web, are primary producers, and are used as an enrichment diet for zooplankton such as rotifers, Artemia and copepods. The importance of zooplankton in aquaculture is well established and has been mentioned in considerable detail elsewhere.1-4
Australia is blessed with vast marine water resources, though lacks freshwater sources that are suitable for aquaculture purposes. Unfortunately, much of Australia’s arable lands are subject to secondary salinisation from the agricultural practices of land clearing, irrigation, and the replacement of deep-rooted native perennial vegetation with shallow-rooted annual crops.5 Inefficient irrigation distribution systems, and the reduction in evapotranspiration that have resulted from land clearing have produced rising water-tables and salt mobilization, decreasing the fertility and economic return of the affected lands.6
Historical evidence shows an upward trend in the financial costs and reduction in rural production from salinity. Prime Minister’s Science, Engineering and Innovation Council of Australia (PMSEIC).7 identified that the area damaged by salinity was estimated to be 4.5% of currently cultivated land, at an annual cost of $130 million in lost agricultural production, consisting of around $90 million in damage to valuable land resources, and at least $40 million in loss of environmental assets. PMSEIC .7 acknowledges that it is inevitable that the consequences caused by salinity will get worse because land use decisions have already been made and are counterproductive in trying to deal with this issue. Later, National Land and Water Resources Audit (NLWRA).8 reported that Australia had approximately 5.7 million hectares of agricultural and pastoral land at high risk of being affected by secondary salinisation, with an estimated 17 million hectares at high risk by 2050. Further to these problems are ecological indications that Australia is experiencing increases in the salinity of underground bore water. Examples are the Deutgam (which serves the Werribee irrigation District) and the Koo Wee Rup (which serves the Westernport region) aquifers found by Boland & Toulmin .9 to be at risk of saline intrusion. This will limit the potential level of extraction for freshwater crop use, but at the same time provides considerable opportunity for utilisation by aquaculture enterprises.
In response to this issue, research has been undertaken into the use of inland saline groundwater for the aquacultural production of finfish, crustaceans, molluscs and seaweed.10-17 but, significantly, little information has been produced on the production of commercially important live feed species for aquaculture in inland saline groundwater.
With this in mind, the aim of this study is to test the hypothesis that commercially important live feed species can be successfully grown in inland saline groundwater by quantifying the growth patterns that are observed. This investigation also seeks to offer recommendations for diversification with regards to the use of saline water for rural farmers that are affected by irrigation or dryland salinity, as well as adding to information concerning the salt water preferences of aquaculture is for the culture of various live feed organisms, as listed in Allan.18 Pillay & Kutty.19 and Smith & Barlow .20 This study takes these examples further and looks at the capacity of inland saline groundwater to be a viable and alternative medium in the production of live aquaculture food items.
Four experiments, coded T1 (Artemia), T2 (Cyclopoid copepod, Cyclops spp), T3 (rotifer Brachionus plicatilis) and T4 (microalgae Nannochloropsis oculata) were conducted as part of this research. The algae used in this experiment (both to act as a test organism and as an enrichment diet for the zooplankton populations) were obtained pre-experiment from the CSIRO (Australian National Algae Collection Centre, Hobart, Tasmania). These were strains of the algae Dunaliella tertiolecta, Nannochloropsis oculata, Pavlova salina, and Isochrysis spp.
Research design and experimental set up
The experimental design in each Trial (1, 2, 3 & 4) featured trials run intriplicate in each of the three saline waters. Aquaria units were assembled in a pattern that was completely random. During the experiment, abiotic variables such as pH (Hanna Instruments, USA), ammonia-nitrogen(Aquarium Pharmaceuticals, USA), salinity (SR6-Salinity meter, Vitalsine, China), temperature, (Hanna Instruments, USA), dissolved oxygen (OxyGuard Handy Polaris Dissolved Oxygen Meter) levels and lighting (Electronics Light Meter No. 05132201) were measured and kept in a balanced condition so that the study highlighted the merits of the different waters. The starting point for salinity was 25ppt with a pH of 8.5. The individuals in the experiment were the live food species used for aquaculture. The treatment waters consisted of seawater, artificial sea water and saline groundwater. The response variable was the size and density of algal colonies and zooplankton at the end of the study.
Description of saline water and chemical properties
The saline groundwater with a salinity level of 25ppt was sourced from Donald (a small town in central Victoria approximately 283 km north west of Melbourne, Australia; location 36°22′13.07″S, 142°58′56.68″E). Sea water of 33ppt salinity was obtained from Seaholme (a coastal town approximately 12 km west southwest of Melbourne on the north western shore of Port Phillip Bay; location 37°52′11.94″S, 144°50′33.32″E). Artificial seawater was prepared using a commercial artificial seawater mix (Prodac Ocean Fish, Prodac International, Cittadella, Italy), with tap water being added to arrive at a salinity of 25ppt. This product was used because of the quoted 99% grade of purity, and because it is presumably representative of a typical commercial artificial seawater mix. Chemical composition of groundwater and seawater, namely Sodium (Na+), Potassium (K+), Calcium (Ca2+), Magnesium (Mg2+), Sulphate (SO42-) and Chloride (Cl-) content, were determined with an Atomic Absorption Spectrometer (AAS) (Varian AA20, Melbourne), and quantitative analysis was further conducted by undertaking titrations where applicable and determination of total dissolved solids (TDS) was undertaken according to the protocols described by APHA- AWWA-WEF .21
Counting method
Before counting, all samples were homogenised to ensure that random sampling error would not be minimised. This was done by giving the container a swirl and applying aeration to make sure that the biomass of algae or zooplankton were evenly distributed through the mix. Cellular density of algae was determined by counting three aliquots of cultures using a Neubauer Haemocytometer, while zooplankton samples were counted by collecting a 1mL sample at random, which was then distributed in droplet form onto a petri dish. Samples were then viewed through a dissection microscope (Leica, China) with counting assisted with a hand counter (Compass, Taiwan). Three samples consisting of 1mL each for a grand total of 3mL were then counted, and an average of the three readings weretaken and percentage error was determined.
Experimental procedure for Artemia salina (T1)
The method described in APHA- AWWA-WEF .21 was used to measure the moisture content of the Artemia eggs (Salt Creek Inc., Salt Lake City, Utah, USA). Hatching the Artemia eggs followed the techniques described in Daintith.22 Nauplii were separated from the eggs using a homemade apparatus made from a 1.5lt plastic drink bottle and rubber tubing. With the bottle held in an inverted position and tubing pushed through the lid by 25mm, a concentrated batch of pure nauplii was collected.
Algae feed was administered equally to all units over the experiment. The estimation of the Artemia biomass was established at 270,000 and distributed at 30,000 per aquaria. (Standard deviation = 1031.98, % of error 3.55).
Experimental procedure for cyclopoid copepod (Cyclops spp) (T2)
Copepod collection method
The population of copepods used in this experiment was obtained with the use of a zooplankton net with an 80µm mesh size (Australian Filter Specialists, NSW). Several sweeps across and through the surface layers at Seaholme in Port Phillip Bay was able to provide suitable biomass for the purposes of the experiment. The catch of copepods were then allowed to sit in a lightly aerated, dark coloured tub for one week prior to use in the experiment.
Two feeding diets were used for the copepods. Diet one was formulated from a feed used by Lee et al.23 Diet two consisted of Isochrysis spp algae. The combined use of two food types for copepods was adopted, as suggested by Rhodes & Boyd .24 Estimation for the copepod biomass was established at approximately 81,000 individuals distributed at 9000 individuals per aquaria. (Standard deviation = 409.26, % of error 4.68).
Experimental procedure for rotifers Brachionus plicatilis (T3)
Rotifer cultures for this study were obtained through Fuji Fuels (a biofuels company based in Thomas town in the northern suburbs of Melbourne), and were maintained using miscellaneous techniques as described in other studies.22,25-26 Rotifer biomass was estimated to be approximately 72,000 individuals, which were distributed at around 8,000 per aquaria (standard deviation=217.94, % of error 3.02). Algae Nannochloropsis oculata spp was used as the basic enrichment diet and inoculated into aquaria prior to the introduction of rotifers to ensure the presence of a readily available food source.
Experimental procedure for Nannochloropsis oculata (T4)
Nannochloropsis oculata was inoculated into all nine carboys at an initial count of 2.89 × 10-4ml-1 from pre- stock cell counts made using a compound light microscope (Prism/Optical, Australia) with a Neubauer haemocytometer slide (Marienfeld, Germany).
Statistical analysis
All data were recorded for Hypothesis testing on Microsoft Excel (Seattle, Washington) spreadsheets (2007) for subsequent data analysis of by using analysis of variance (ANOVA) and F-test statistic at α = 0.05.
The results of preliminary analysis of all waters utilized in these experiments are shown in Table 1.
|
Parameters |
Sea |
Artificial |
SG/W |
|
Concentration (mg/L-1) |
Concentration (mg/L-1) |
Concentration (mg/L-1) |
|
|
Na+ |
10930 |
10752 |
7580 |
|
Mg2+ |
1684 |
1295 |
1330 |
|
K+ |
410 |
390 |
90 |
|
Ca2+ |
370 |
416 |
293 |
|
SO₄²⁻ |
3500 |
2701 |
2700 |
|
Cl⁻ |
19640 |
19345 |
13600 |
|
% TDS |
3.69 |
n/a |
2.58 |
Table 1 Chemical analysis of Prodac Ocean Fish artificial sea water and inland saline groundwater, using Varian AA20 Atomic Absorption Spectrometer.
Figure 1 shows the growth characteristics of Artemia salina in the three saline waters and indicates that there are no significant differences between each of the waters being tested (P-value of 0.828, > α=0.05). Estimation of moisture content in Artemia cysts was found to have a mean value of 8.55%, with a mean dry weight of 0.4185g.
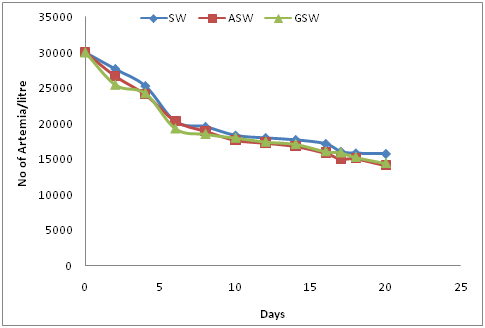
Figure 1 Growth of Artemia in Sea Water (SW), Artificial Sea Water (ASW) and Saline Ground Water (SGW).
Three samples were taken at random from the three waters and viewed through a dissection microscope at the same magnification. Figure 2a demonstrates the obvious visual differences in the growth rates of Artemia from each of the different water types that were tested as part of this investigation.
Uniformly smaller bodies were observed from the artificial sea water aquaria. They evidently fed, as indicated by the food matter that was readily visible in the gut. The implication here is that the artificial sea water has inhibited the growth rate of individual Artemia but not fecundity and consequent rate of population increase.
The body sizes in the seawater tended to be more uniform and larger than the bodies depicted in the photo from the artificial sea water (Figure 2b). Being larger, the body colour tended to be darker, possibly as a consequence of having more food in the gut.
There tended to be considerably more larger-sized bodies in the groundwater aquaria than the other two experimental water types that were tested, though the body sizes were not uniform and variation was considerable (Figure 2c). With such variety in body sizes being observed, it is possible that more population activity was occurring within these aquaria.

Figure 2 (a) Smaller and lighter coloured Artemia grown in artificial sea water, (b) uniform body size of Artemia grown in sea water; (c) differing body sizes of Artemia grown in SGW.
Growth in cyclopoid copepods (T2)
Initially all animals in each water type tested showed a sharp decrease in numbers. Figure 3 shows how the copepod biomass returned to measurable numbers after a very poor start, during which numbers were observed to decline significantly. The copepod densities that were observed between each of the waters being tested varied at a statistically highly significant level (P-value 0.0001, >α = 0.05).
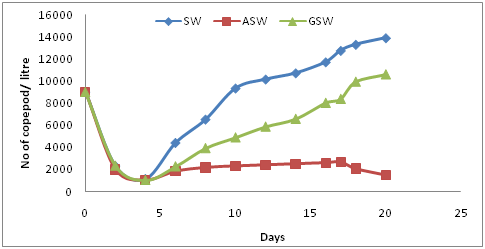
Figure 3 Growth of copepods in Sea Water (SW), Artificial Sea Water (ASW) and Saline Ground Water (SGW).
A series of photographs taken in the laboratory of Cyclopoid copepods revealed an interesting situation with regards to the coloration being adopted by the exoskeleton of these animals. When viewed under a microscope, (a Motic BA300 microscope with Modicam 2000 digital camera), a typically orange-brown colour (Figure 4a) that is characteristic of the body of this species was evident on the hollow exoskeleton, which represented the animal’s first moult post-harvest from the sea. This can be compared with the colour of the post moult copepod (Figure 4b), where the pigment of its former colour has significantly changed to this glass-like appearance.
In another situation (Figure 4c), a female copepod demonstrated that the animals had adapted to a changed environment as much as necessary to commence breeding. This situation may indicate that the Cyclopoid copepod may become more fecund with each generation, and that a certain level of domestication could be expected to develop that could lead to improved survival rates, but this is subject to confirmation by further experimentation.

Figure 4 (a) Cyclopoid exoskeleton showing orange brown colour of wild harvested copepod. (b) Post moult Cyclopoid copepod from T2, saline groundwater; (c) Female copepod (from saline groundwater) with newly hatched naupli.
Growth in rotifer Brachionus plicatilis (T3)
Figure 5 outlines the rate of rotifer growth in the three saline waters (P-value 0.00 < α=0.05). The statistical analysis for T3therefore demonstrated that the rotifer Brachionus plicatilis differed in terms of density depending on the water that it was being grown in. The low P-value with an F-statistic of 22.72 provides strong evidence against the H0 hypothesis.
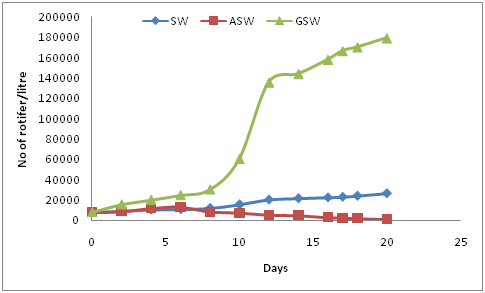
Figure 5 Growth of rotifer in Sea Water (SW), Artificial Sea Water (ASW) and Ground Saline Water (GSW).
Growth in Nannochloropsis oculata (T4)
The performance outline of the three saline waters is shown in Figure 6. Statistical analysis for T4 reveals a P-value of 0.067 >α = 0.05. This P-value for N.oculata means that the data are not statistically significant at the α = 0.05 level. However the 2.99 analysis of variance F statistic shows that there was a statistically significant difference in the mean values for saline groundwater over artificial sea water.
The first feed items taken by larval fish is often restricted by the gape of their mouths.27 since food particles cannot exceed this size, even micro-pelleted diets may be unsuitable for fish and cannot be expected to provide them with the sustenance required to get them through the crucial early life stages.28-30 The size of rotifers and early-instar Artemia and copepod species are generally small enough to be detected and devoured by larval fish species, explaining their attractiveness as a live feed diet for a multitude of fish species.31-33 As this study has demonstrated, the breeding of live feed species can be achieved using saline water that just happens to be quite well suited to their needs.
In the experiment T1 (featuring Artemia salina), darker orange/brown coloured bodies were first noticed in the groundwater aquaria. As this colour difference between the waters was short-lived, the cause was thought to be more from a shorter lag period in the reproductive rate of the Artemia when placed in saline groundwater, as opposed to being from any specific ionic water parameters per se. Random photos, taken towards the end of the T1 Artemia trial, appeared to show the animal with a full gut, indicating that the animals had fed normally. These photos (Figure 2) also represent a point of difference in the average growth sizes seen from the different water types tested. Smaller and lighter coloured Artemia developed within the artificial seawater. Further study could be conducted on this point to see if this characteristic had any influence on a longer life span, as well as other important aspects, such as fecundity and overall productivity levels.
Relatively uniform body sizes of Artemia were observed in the seawater aquaria. As the size of a larval fish’s mouth is a major limiting factor in feeding, this uniform consistency in size becomes a considerable and desirable advantage in a live feed situation. Extra quantities of larger bodied Artemia were observed in the GW, which could potentially present issues with situations in which various instars are being offered to very small larvae, such as those produced by barramundi, Lates calcarifer, which are only 1.5mm long, and therefore require necessarily small (and preferably uniform) Artemia larvae upon which to feed.34-36 This factor relates to the early observation of darker bodies and indicates that the Artemia have adapted somewhat sooner to the groundwater.
The growth performance of copepods (Figure 2) and their initially poor performance, followed by an explosive population rebound, are in agreement with research carried out by Conseic et al.1 who described various difficulties in copepod culture and survival, including the finding that greater success was initially found for second and third generation cultures. In this trial, a mistaken observation was made that the cultures had crashed because only dead orange/brown carcasses were found. One possible reason why the culture could have been thinned out so dramatically could have been cannibalistic tendencies, as indicated by Rhodes & Boyd .24 and Lee et al.23 At a later date, closer inspection revealed that while where there was much mortality; there were a reasonable number of hollow moulted exoskeletons. The authors’ previous experiences with yabbies (the Australian native freshwater crayfish Cherax destructor) have shown how the fresh moult can be mistaken for the whole animal.
Identification became obscure, as post-moult, the colour of the copepod body was near transparent or light grey, which displayed the internal organs in black. The experimental units were never shut down and as the copepods reappeared, population counts resumed.
Even though the statistics say that there was no significant difference in using either of the salt water types, the preference of this study would be to use seawater. The reason for this is due to the delicate nature displayed by the animal in managing translocation from the catch-point and introduction into the culture water. The opinion of the authors from this study is that the population would be lost using either the groundwater or artificial seawater at these early stages. Comparable findings were made by Conseic et al.1 which identify long generation times with some copepod species, as well as sensitivity to alterations in parameters such as hardness, salinity and temperature.
Rotifer growth in seawater and artificial seawater were slow by comparison to the immediate acclimatization displayed in the GW aquaria. Growth in the groundwater developed at such a rate that all food was ingested overnight, leaving the water almost clear. Rising NH3 levels were a further indication of the increasing growth activity occurring in the groundwater aquaria, presumably due to the metabolic breakdown of protein and other substances that led to the formation and liberation of NH3. While there was a point of difference in population numbers, no visible differences were observed in body size or colour of the animals from any of the units, after what would have been the second or third generations.
In order to test and determine the suitability for supporting Nannochloropsis oculata growth for each water type, inoculations were staggered and time was given for the cultures to develop. After twelve days, sufficient green water had developed to determine biomass accurately. Growth in the artificial sea water units achieved half the productivity of the seawater units. Seawater performed consistently, whereas the growth produced from the GW was considerably better (the extent to which was somewhat surprising). Two main reasons for explaining the groundwater results would be that N. oculata is a suitable algae species in groundwater, while other candidate species, such as Dunaliella tertiolecta, Pavlova salina, and Isochrysis spp. may have to be tested first to ascertain suitability with this water type. Also, the combination of macro and micro nutrients of the groundwater were better assimilated by N. oculata. With the sea ater being used in its raw state, pollution within Port Phillip Bay could have also been a contributing factor, thought this would have to be tested and confirmed to be able to pass judgment on this issue.
Aquaculture is earmarked as a growth industry for future sustainable food production and this project concentrates on an important element of that endeavor. Not only is this research relevant to regular practices within the aquaculture industry, but it aims to provide solutions for the use of inland areas that have been compromised by high salinity levels. Alternative farming methods are now a fact of life for many farming communities affected by dry land salinity. As the improvement of the land and water quality is often a slow and gradual process, some local farming communities have dwindled in numbers and health status due to economic hardships.37
Aquaculture is heralded as a sustainable solution to providing income and industry growth. It is hoped that the results of this experiment and those that are similarly aligned could influence future planning with regards to aquaculture development. In Australia, as well as other parts of the world, land that is no longer suitable or viable for agricultural farming may be very suitable for aquaculture. The analysis of groundwater could yield vital information as to specific suitability towards a number of potential aquaculture harvests, whether they consist of algae, live feeds or finfish.
The current study seeks to embrace the basic principles of ecological sustainability and biodiversity, as well as those of economic management, by demonstrating the ability to adapt to a changing world. It is hoped that the positive outcomes that result will influence various levels of aquaculture practice in future years.
As a global example, Israel now has to deal with both dry land salinity and government-imposed water restrictions due to compromised land and water scarcity, situations that are exceedingly familiar to Australian farmers due to a fundamentally El-Nino/La Nina-based climatic system.38 Many authors.8,10,38 indicate that this is becoming common in many parts of the world. Crop farmers invariably experience difficulties in sowing a crop, or produce crops of poor yield because of dry land salinity or increasing soil salinity that has stemmed from irrigation practices. One method of dealing with this problem would be the pumping and disposal of saline groundwater but this cost would be prohibitive to many small rural towns.39 The topography of northern and southern Israel is somewhat similar to that of Mildura and Shepparton respectively, so there should be some scope for utilizing similar principles to develop aquaculture in this area.40
Integration of aquaculture with agriculture has also become increasingly attractive.41 as there is a greater emphasis being placed on re-using resources such as land and water. This in turn leads to the development of guidelines for managing and controlling saline groundwater.20 which must be a major initiative well into the future.
This study has demonstrated that there are potentially some very good opportunities for the utilisation of inland saline groundwater for live feed production and associated aquaculture activities. ar advantages in the use of inland saline ground water for aquaculture have been proven in this study, with rotifers of the species Brachionus plicatilis and the algae Nannochloropsis oculata being particularly suited to the chemical composition of the water being used in the study. It could easily be argued that inland saline aquaculture clearly provides an option for diversification for agricultural holdings, but a sobering element that must be remembered is that the inland saline industry has been examined for suitability for the past two decades with only moderate success that has yet to be commercially realised to any great extent. Kolkovski.42 indicates that a good number of inland aquaculture farmers have already given up on inland saline aquaculture to look elsewhere, but it is hoped that by approaching aquaculture from the base of the food pyramid (i.e. With algae and zooplankton culture), more farmers can be encouraged to stay the course and develop hatcheries or grow out systems that are capable of supporting thriving enterprises. This way, the skills learnt from zooplankton production can be developed, and eventually enable a fish hatchery to be developed. This foundation then paves the way for the business to move in an upward direction. This can then develop into the growing out of fish stocks for markets.
The overall majority of reports pertaining to inland saline aquaculture are about the production of fish or trialling suitable euryhaline fish species for this form of aquaculture. In other words, the building blocks of the industry were never established, as the prime focus appears to have only been on the end result. The fundamental reality of this top-down approach to building an industry is that no long term stability can be established, and that it has little or no diversity to fall back on in times of adversity.
Few inland saline aquaculture businesses have been successful (with very little work conducted outside of experimental work) and at present there is limited focus on the production of varieties of microalgae or zooplankton.42 Markets exist for live feeds in aquaculture, and therefore research into their exploitation would require some work, but there are many opportunities here that could be exploited, remembering that live feeds effectively underpin hatchery operations, and are capable of providing a significant income stream. It is hoped that by supplying technical information that can be reproduced by aqua culturists, the development of the base of the aquatic food pyramid can take place in inland saline aquaculture situations, thus providing a considerable potential boost to the industry.
We are deeply indebted to Mr. Ben Hokinand Fuji Fuels, Thomastown, for providing some of the materials that were relied on in this investigation.
None.

©2016 McDowall, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.