Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 1
1Laboratory of Plankton Research, Department of Fisheries Management, Bangladesh Agricultural University, Bangladesh
2Department of Arts, Education & AgriTech, Melbourne Polytechnic, Australia
3Bangladesh Fisheries Research Institute, Bangladesh
Correspondence: Saleha Khan, Department of Fisheries Management, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh
Received: January 02, 2024 | Published: February 21, 2024
Citation: Ahmed S, Ritu JR, Khan S, et al. Influence of the green microalga, Chlorococcum sp. on the growth of freshwater rotifer, Brachionus calyciflorus pallas. J Aquac Mar Biol. 2024;13(1):8-13. DOI: 10.15406/jamb.2024.13.00390
The success of the aquaculture sector relies on a consistent supply of healthy fish seeds. Rotifer has been identified as superior live food to artificial feed for nurturing fish larvae, the culture development of which largely depends on green microalgae. This study aimed to investigate the suitability of Chlorococcum sp. for enhancing the production of Brachionus calyciflorus. Two experiments were conducted to assess the effects of different food types and concentrations of Chlorococcum sp. on the growth of B. calyciflorus. In the first experiment, three food types were tested: live Chlorococcum sp. (1 x 105 cells/mL; T1), baker’s yeast (0.2 gm/L; T2), and a combination of live Chlorococcum sp. and baker’s yeast (0.5 x 105 cells/mL + 0.1 gm/L; T3). The highest population density and growth rate (r) of rotifers were observed in the T1 diet. In the second experiment, three concentrations of Chlorococcum sp. were tested: 0.5 x 106 cells/mL (T1), 1 x 106 cells/mL (T2), and 3 x 106 cells/mL (T3). Both the population density and growth rate of B. calyciflorus were found highest in the T3 diet. In conclusion, Chlorococcum sp. at a concentration of 3 x 106 cells/mL is suggested as the best food for the successful mass culture of the rotifer B. calyciflorus.
Keywords: Brachionus calyciflorus, Chlorococcum sp., culture, growth rate, population density
Aquaculture is currently one of the fast-growing food production industries. To ensure the food and nutrition security of the global population, there is a prime need to make strides in aquaculture, the progress of which largely depends upon the advancement of fish larval-rearing techniques. The availability of sufficient healthy fish seeds is a crucial requirement for a successful fish culture.1 Nevertheless, the major impediment to fish seed production in hatcheries is ensuring a consistent supply of live food for fish larvae. Over the past few decades, rotifers have proven to be the most suitable species for the initial feeding of larvae for numerous commercially important fishes. The mass culture of Brachionus sp. is a key factor in the success of the global aquaculture sector.2,3
Rotifers belonging to the genus Brachionus are commonly used as live food in the rearing of larvae of both freshwater and marine species of fish, shrimps, crabs, prawns, and mollusks. The nutritional value, density, and body size of rotifers all play critical roles in the feeding, development, and survival of larval fish.4,5 Various types of food, including naturally occurring ones like microalgae and chemically prepared diets such as micro-encapsulated pellets have been extensively used for the mass-production of Brachionus sp.6,7 A higher proportion of Brachionus individuals bearing multiple amictic eggs is observed when more suitable food is available.8,9 Baker's yeast is also utilized in the large-scale production of Brachionus spp.10 which further unveiled the poor nutritional quality of yeast-fed rotifer.11 Among various feed types, microalgae have been predominantly used as food in rotifer cultures.12,13 Under both field and laboratory conditions, diverse microalgae are used for feeding planktonic rotifers because producing microalgae on a large scale is substantially less expensive than producing other prepared meals.14,15
Microalgae are frequently used for zooplankton rearing because of their ability to produce higher biomass in a short period and their enriched nutritive profile.16–18 One of the most often employed types of microalgae for feeding larval fish and zooplankton is the green microalgae from the Chlorophyceae family. Among them, Chlorococcum sp. is a green freshwater microalga that is considered one of the highest carotenoid producers including astaxanthin, lutein, zeaxanthin, neoxanthin, canthaxanthin, and violaxanthin that may have great significance in aquaculture industry.19–21 Chlorococcum sp. was examined for its potential to inhibit cholinesterase and for its antioxidant properties.22 Chlorococcum sp. can produce astaxanthin, which includes several critical metabolic capacities counting upgrade of safe reactions and security against infections such as cancer by rummaging oxygen-free radicals.23 Astaxanthin-producing microalgae are also broadly utilized as a shade-added substance within the aquaculture industry.23,24 Consequently, for the desirable growth of rotifers, the green microalga Chlorococcum sp. may be a viable live food source.
Numerous biotic and abiotic factors have significant effects on rotifer density.25 The number of rotifers is significantly influenced by biotic elements such as phytoplankton diversity and concentration.12 Laboratory feeding trials of B. calyciflorus exhibit increased algal intake with an increase in feed concentration until an asymptote is reached.25,26 Microalgal species broadly used in rearing rotifers are namely Chlorella ellipsoidea,27 Chlorella sp., Scenedesmus obliquus,25,28,29 Scenedesmus acutus,12 Chlorella vulgaris,12–14,30 Aurantiochytrium sp., Isochrysis sp., Nannochloropsis sp.31 and Monoraphidium contortum.32 The effect of astaxanthin-producing green microalga Chlorococcum sp. on the culture and growth of B. calyciflorus has not been studied yet. With that in mind, the effects of green microalga Chlorococcum sp. on the growth rate and population density of rotifer B. calyciflorus were investigated.
Collection and culture of the microalga and the rotifer
The previously maintained pure strain of the green microalga Chlorococcum sp. in the “Laboratory of Plankton Research, Department of Fisheries Management, Bangladesh Agricultural University, Mymensingh” was used for this study (Figure 1). Chlorococcum sp. was mass cultured in Bold Basal Medium (BBM) (Figure 2). Microalgal cells in the log phase of growth were harvested, centrifuged at 3000 rpm for 7 minutes, rinsed with distilled water, and resuspended in moderately hard water that was prepared by dissolving 96 mg of NaHCO3, 60 mg of CaSO4, 60 mg of MgSO4, and 4 mg of KCl in one liter of distilled water (EPA medium).33
The rotifer species Brachionus calyciflorus was isolated from the backyard pond of the Faculty of Fisheries, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh. Chlorococcum sp. was used as food to successfully culture the rotifer Brachionus calyciflorus.
Crude protein and crude lipid determination of Chlorococcum sp.
Crude protein and crude lipid content of Chlorococcum sp. were analyzed in triplicates by following the method of Ritu et al.16
Two experiments were conducted to investigate the effects of different food types and cell concentrations of Chlorococcum sp. on the population density and growth rate of B. calyciflorus (Figure 3). In the first experiment, the effects of live Chlorococcum sp. (1 x 105 cells/mL; T1), baker's yeast (0.2 gm/L; T2), and a combination diet of baker's yeast + Chlorococcum sp. (0.5 x 105 cells/mL + 0.1 gm/L; T3) on the growth of the rotifer B. calyciflorus was examined. In the second experiment, the impact of three different concentrations of the green microalga Chlorococcum sp. on the growth of the rotifer B. calyciflorus was examined. The concentrations of the microalgae given as feed to the rotifers were 0.5 x 106 cells/mL (T1), 1 x 106 cells/mL (T2), and 3 x 106 cells/mL (T3). In both tests, rotifers were grown under usual conditions in 20 L container using EPA medium with an initial density of 6 individuals/ mL, and the food was given two times a day – at 8:30 AM and at 8:30 PM. During the experiment, the room temperature was 25 ± 2˚C. By obtaining 2-3 aliquot samples from each container the density of the rotifers was estimated every day under a microscope (B-510BT OPTIKA, Italy) at a magnification of ×10. After 12 days, the experiment was closed since the quantity of B. calyciflorus started to decrease. Based on the collected data, the rate of population increase (r) was calculated using the exponential growth equation as follows:
Where, = initial population density, and = density of population after time (days).34 The value of was obtained from a mean of 4-5 values during the exponential phase of the population growth in each treatment.
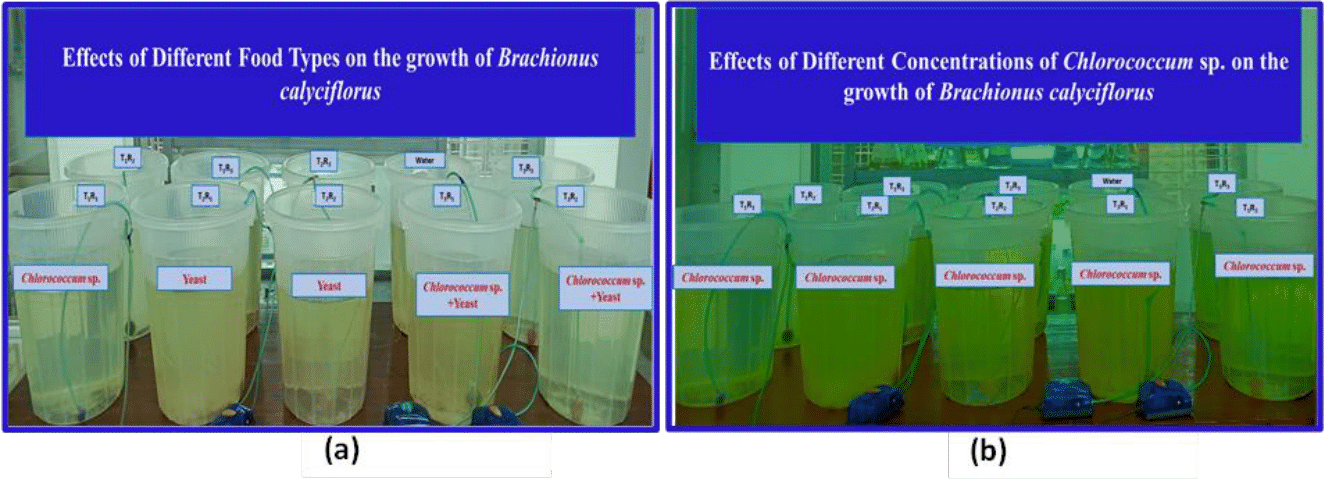
Figure 3 Experimental setups of Brachionus calyciflorus culture: (a) Feed given in Experiment 1 [T1 = Live Chlorococcum sp. (1 x 105 cells/mL), T2 = Baker’s yeast (0.2 gm/L), and T3 = Live Chlorococcum sp. 0.5 x 105 cells/mL + Baker’s yeast 0.1 gm/L, and (b) Feed given in Experiment 2 [ T1 = 0.5 x 106 cells/mL, T2 = 1 x 106 cells/mL, and T3 = 3 x 106 cells/mL of live Chlorococcum sp.].
Statistical analysis
The data were analyzed using one-way ANOVA (SPSS 25). Significant differences among means were determined using Duncan’s multiple range test (DMRT). Differences were considered significant at P˂ 0.05.
Crude protein and crude lipid content of Chlorococcum sp.
On a dry matter basis, the crude protein and crude lipid content of Chlorococcum sp. were 30.5% and 18.2%.
Experiment I
Experiment I
The population growth curve of the rotifer, B. calyciflorus in response to three different feed treatments (live Chlorococcum sp., baker's yeast, and a combination diet of live Chlorococcum sp. + baker's yeast) is shown in Figure 4. The food types resulted substantial impact on the rotifer's maximal population density. When Chlorococcum sp., baker's yeast, and a combination diet of live Chlorococcum sp. + baker's yeast were fed, the highest population densities of B. calyciflorus were found as 255 ± 11, 182 ± 14, and 72 ± 4 individuals/mL in T1, T3, and T2, respectively (Figure 4). In T2 where yeast was used as feed, the rotifer population peaked on the 7th day of the culture period. The highest population densities of the rotifer were found on the 8th day of the culture period where the live Chlorococcum sp. (T1) and a combination diet of live Chlorococcum sp. + baker’s yeast (T3) were fed. Among the three treatments, B. calyciflorus showed the best growth in terms of population density in T1 where live Chlorococcum sp. was fed (Figure 5).
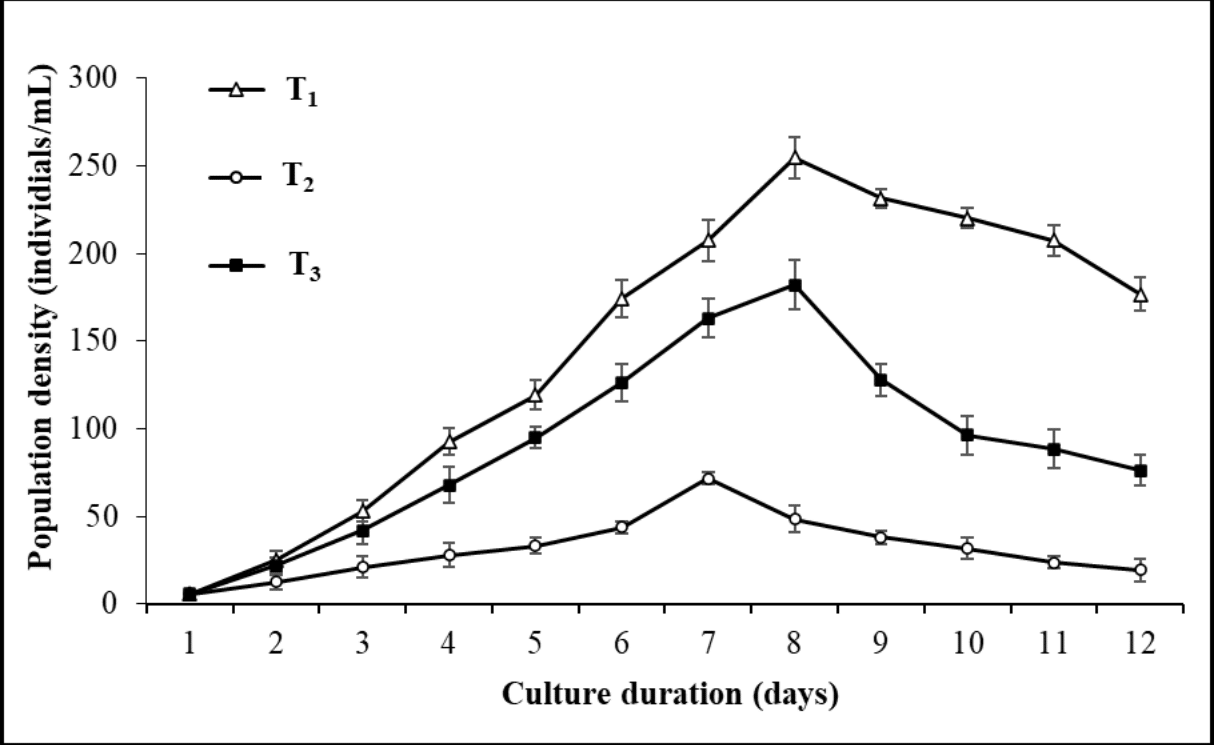
Figure 4 Population density of freshwater rotifer, Brachionus calyciflorus (individuals/mL) fed on different diets (T1 = Live Chlorococcum sp. 1 x 105 cells/mL; T2 = Baker’s yeast 0.2 gm/L; T3 = Live Chlorococcum sp. 0.5 x 105 cells/mL + Baker’s yeast 0.1 gm/L). Each point and vertical line represents mean ± SD for three replicates.
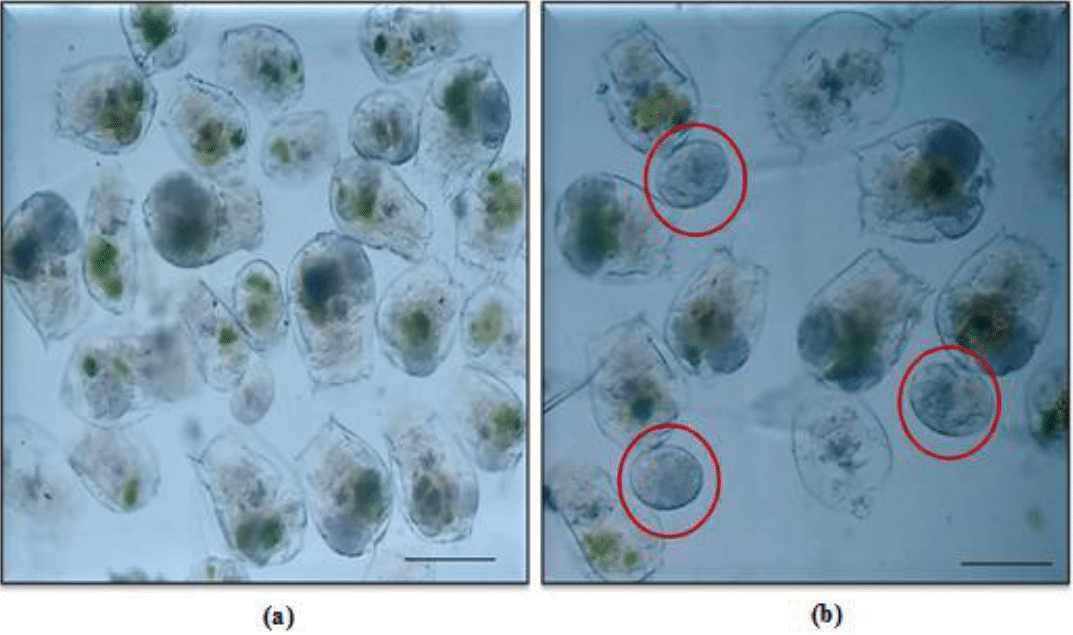
Figure 5 Micrographs showing (a) Chlorococcum fed individuals of Brachionus calyciflorus, and (b) Eggs of B. calyciflorus within red circles. Scale bar: 100 µm.
Food type showed a highly significant (P< 0.05) impact on the population growth rate (r) of B. calyciflorus (Figure 6). The food types considerably altered the population growth rate (r) for B. calyciflorus, which was 0.587 ± 0.01, 0.35 ± 0.02, and 0.53 ± 0.01 day-1 in T1 (live Chlorococcum sp.), T2 (baker’s yeast), and T3 (a combination diet of live Chlorococcum sp., + baker’s yeast) respectively.
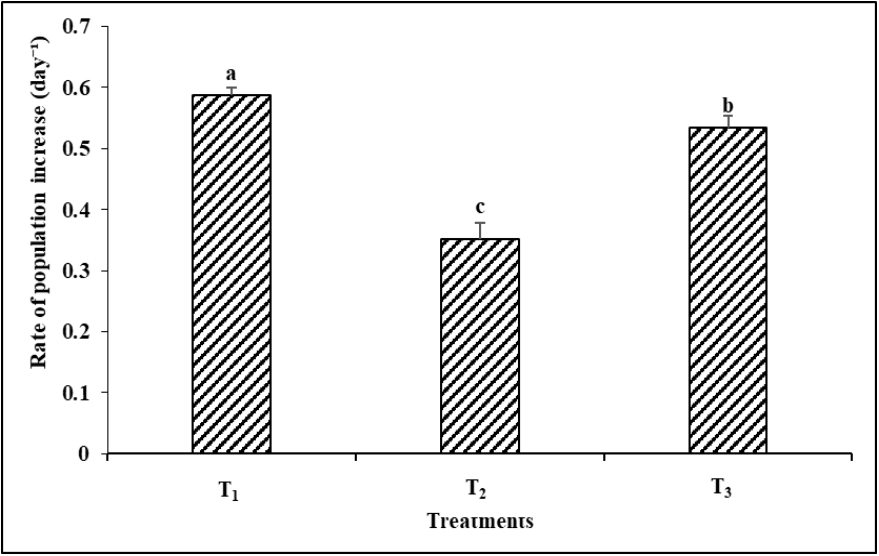
Figure 6 Rate of population increase (r) per day for Brachionus calyciflorus in relation to/ with different food types. (T1 = Live Chlorococcum sp. 1 x 105 cells/mL; T2 = Baker’s yeast 0.2 gm/L; T3 = Live Chlorococcum sp. 0.5 x 105 cells/mL + Baker’s yeast 0.1 gm/L). Each bar and vertical line represent mean ± SD for three replicates. Means with different letters are significantly different from one another (P< 0.05).
Experiment II
The population growths of the rotifer, B. calyciflorus raised in three different concentrations of Chlorococcum sp. are shown in Figure 7. The microalgal concentration significantly (P< 0.05) impacted the rotifer population density. The highest rotifer density of 128 ± 6 (in T1), 255 ± 11 (in T2), and 399 ± 5 individuals/mL (in T3) were found on the 7th, 8th, and 10th day of the culture period respectively (Figure 7). Rotifer growth began to decrease after the 7th day of culture in treatment where a concentration of 0.5 x 106 cells/mL of Chlorococcum sp. was given as feed. At 1 x 106 cells/mL microalgal concentration, rotifer development began to decrease from the 9th day. In T3 where 3 x 106 cells/mL of Chlorococcum sp. was given as feed, the maximum density of the rotifer was obtained on the 10th day and then it began to decrease from the 11th day.
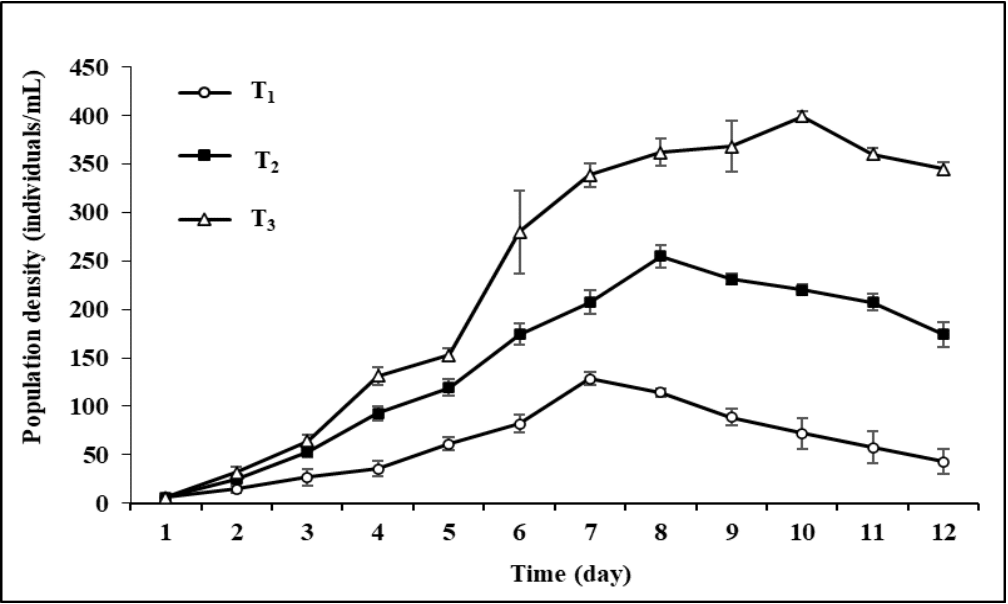
Figure 7 Population density of freshwater rotifer, Brachionus calyciflorus (individuals/mL) fed on different concentrations of Chlorococcum sp. (T1 = 0.5 x 106 cells/mL; T2 = 1 x 106 cells/mL; T3 = 3 x 106 cells/mL). Each point and vertical line represent mean ± SD for three replicates.
The highest population growth rate (r) of B. calyciflorus was recorded as 0.66 ± 0.01 day-1 at the concentration of 3 x 106 cells/mL of Chlorococcum sp. which was significantly higher (P<0.05) than other food types (Figure 8). The growth rate of the rotifer was 0.59 ± 0.01 and 0.45 ± 0.03 day-1 when fed 1 x 106 cells/mL and 0.5 x 106 cells/mL of Chlorococcum sp. respectively.
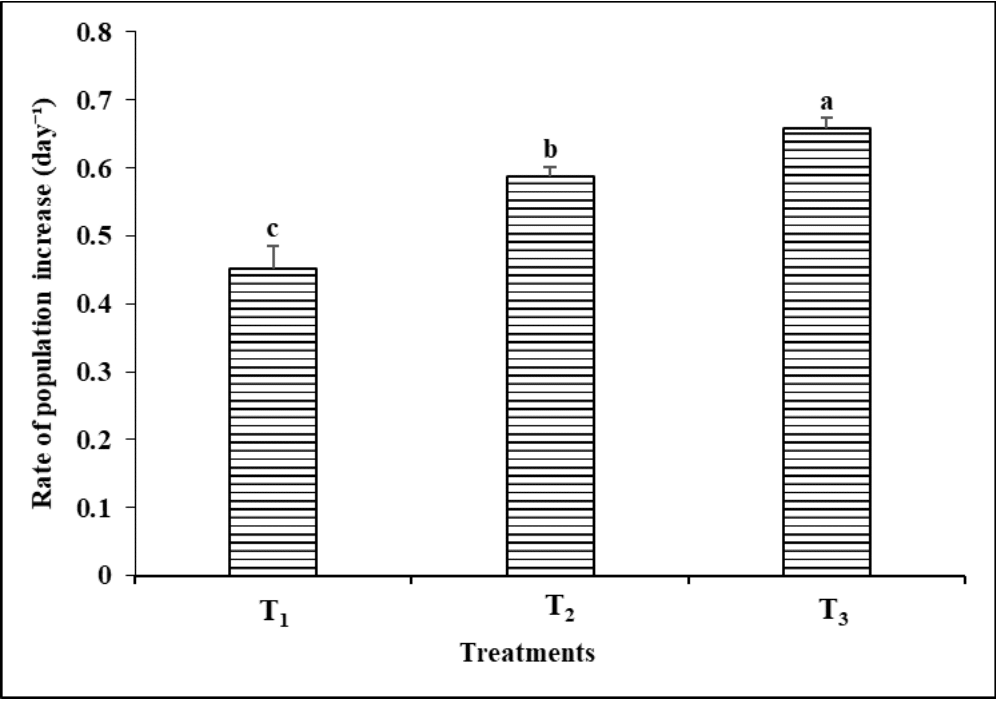
Figure 8 Rate of population increase (r) per day of Brachionus calyciflorus in relation to different cell concentrations of Chlorococcum sp. given as feed (T1 = 0.5 x 106 cells/mL; T2 = 1 x 106 cells/mL; T3 = 3 x 106 cells/mL). Each bar and vertical line represent mean ± SD for three replicates. Means with different letters are significantly different from one another (P< 0.05).
Both feed type and feed concentrations have remarkable effects on the population growth rates of rotifers.13 In the first experiment, three treatments including live Chlorococcum sp., baker’s yeast, and a combination diet of live Chlorococcum sp. + baker’s yeast were used in the culture of the rotifer Brachionus calyciflorus. The population growth of the rotifer B. calyciflorus was found higher (255 ± 11 individuals/mL) in T1 where they were fed only live Chlorococcum sp. followed by 182 ± 14 individuals/mL in T3 (a combination diet of Chlorococcum sp. and baker's yeast) and 72 ± 4 individuals/mL in T2 (only baker’s yeast). Compared to other diets the live Chlorococcum sp. was found best for the growth of the rotifer B. calyciflorus which is supportive of the findings of Akter et al.27 The highest population density of B. calyciflorus was reported as 28.6 ± 5 individuals/mL when fresh live Chlorella ellipsoidea was fed27 which was far lower than the present findings. Sarma et al.13 also showed the maximum population density (103 ± 8 individuals/mL) and growth rate (0.63 ± 0.04 day-1) of B. calyciflorus when fed Chlorella vulgaris which is also far lower than the present study. The growth patterns of B. calyciflorus might potentially be influenced by the nutritional value and digestibility of the microalgal cells.14,35,36 On the other hand, in the present study baker’s yeast didn’t show satisfactory growth of the rotifer. Hirayama and Funamoto11 have also stated that baker’s yeast is unstable for zooplankton culture. But when baker’s yeast was supplemented in a combination with live Chlorococcum sp., B. calyciflorus attained higher peak population abundance compared to the rotifer population found in the diet of only yeast. Khatun et al.37 also got satisfactory production of B. calyciflorus (67 ± 8 individuals/mL) when fed a combination diet of yeast with fresh Chlorella vulgaris which is far lower than the population density (182 ± 14 individuals/mL) found in the treatment of combination diet of the present study. Hence, in the controlled laboratory conditions, whether served Chlorococcum sp. solely or as part of a diet that contains yeast, has a positive impact on the population growth of B. calyciflorus. Consequently, despite not being equivalent to Chlorococcum diet, yeast can be employed in rotifer growth systems at low concentrations to complement the needs of algae.
In the second experiment, the concentration of the microalga Chlorococcum sp. as food resulted in a direct impact on the increase of the population density of the rotifer B. calyciflorus agreeing with the findings of several earlier investigations, both from field collections and at laboratory studies.38,39 Rotifers, reported as opportunistic species that respond more quickly to changes in microalgal food levels40 are also confirmed in the present study. The suitability of a microalgal species as food for rotifers is influenced by several elements, including size, shape, motility, digestibility, and nutritional content.41–44 In the present study, with the increasing rate of the concentration of Chlorococcum sp. as a feed from 0.5 x 106 cells/mL to 3 x 106 cells/mL, the population abundance of B. calyciflorus was also increased. A similar result was found by Nhi et al.45 where the population density of B. angularis increased with elevated concentration of the cultured Chlorella sp. as feed. A similar increasing growth pattern with the rise in food levels has been documented in several rotifer genera of the family Brachionidae.13,14,29,39,46 Depending on the amount of microalgal food concentration, the peak abundance of the rotifer B. calyciflorus in the current research varied from 128 ± 6 to 399 ± 5 individuals/mL. The difference in microalgal concentration also significantly changed the population growth rate (r) of B. calyciflorus. The population growth rate of B. calyciflorus rose with increased microalgal feed concentration. The growth rates of B. calyciflorus were 0.45 ± 0.03/day in T1, 0.59 ± 0.01/day in T2, and 0.66 ± 0.01/day in T3 where Chlorococcum sp. was given as feed at the rate of 0.5 x 106 cells/mL, 1 x 106 cells/mL and 3 x 106 cells/mL respectively. The growth rate trends related to the concentration of the microalgal feed given in the present study are similar to the findings of Kenari et al.25 who reported the growth rate of B. calyciflorus as 0.18, 0.42, and 0.51/d when fed Chlorella sp. at the cell concentration of 0.1 × 106, 1 × 106, and 10 × 106 cells/mL respectively, though the growth rates in the present study are higher than those reported by them. These may be due to the variation in the nutritional status of different species of microalgae given as feed to the rotifers or the environmental conditions during the culture period. Finally, it can be said that the green microalga Chlorococcum sp. is a nutritious species enriched with a higher content of crude protein (30.5%) and lipid (18.2%) that can be used for mass-scale commercial production of the rotifer B. calyciflorus. However, repeated outdoor mass culture trials are necessary before the dissemination of the culture technology to the rural fish farmers and hatchery operators.
The findings of the study revealed that the cultured live green microalga Chlorococcum sp. is very suitable for mass culture of the rotifer Brachionus calyciflorus. The population growth of the rotifer B. calyciflorus was found to increase with the increased concentration of the live microalgal feed. Outdoor large-scale mass culture of the microalga Chlorococcum sp. and subsequently the rotifer B. calyciflorus using the microalgae may play a significant role in the enhancement of aquaculture by using both of them as live feed in rearing fish larvae in hatcheries. Further outdoor research is needed before the implementation of the findings at the farm/hatchery level.
Md. Sayem Ahmed: Investigation, Formal Analysis and writing -Original Draft; Jinnath Rehana Ritu: Investigation, Formal Data Analysis, Graphical Abstract Preparation and Writing - Original Draft; Saleha Khan: Conceptualization, Methodology, Supervision, Resources, Project Administration, Fund Acquisition and Writing - Review and Editing; Md Helal Uddin: Writing - Review and Editing; Sadiqul Awal: Writing - Review and Editing; Md. Mahfuzul Haque: Supervision, Writing - Review and Editing; Md Kowshik Ahmed: Formal Data Analysis and Graphical Abstract Preparation, Md. Shahin Alam: Formal Data Analysis and Graphical Abstract Preparation.
The research was conducted under a special financial grant from the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh (Grand number BS 79/MoST) which is gratefully acknowledged. The authors are grateful to the Bangladesh Agricultural University Research System (BAURES) for the help provided in the smooth running of the project.
The author(s) declare that they have no known competing financial or non-financial, professional, or personal conflicts that could have appeared to influence the work reported in this paper.

©2024 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.