Journal of
eISSN: 2378-3184


Research Article Volume 11 Issue 3
1UFR des Sciences Agronomiques, de l’Aquaculture et des Technologies Alimentaires (UFR S2ATA), Université Gaston Berger (UGB), Saint-Louis, Sénégal
2ISRA /Oceanographic Research Center Dakar-Thiaroye, Dakar, Senegal
3Département de Biologie Animale, Faculté des Sciences et Techniques, Université Cheikh Anta Diop de Dakar (UCAD), Sénéga
Correspondence: Mbaye Tine, UFR des Sciences Agronomiques, de l’Aquaculture et des Technologies Alimentaires (UFR S2ATA), Université Gaston Berger (UGB), Route de Ngallèle BP 234 Saint-Louis, Sénégal
Received: December 02, 2022 | Published: December 19, 2022
Citation: Tine M, Diallo O, Ndao PD, et al. Implementation of a microalgae and live prey production system (daphnia) to improve African catfish Clarias gariepinus fry growth. J Aquac Mar Biol. 2022;11(3):139-147. DOI: 10.15406/jamb.2022.11.00348
Fish farming in Senegal encounters many difficulties, especially during the first life stages, which are more demanding for food and more sensitive to variations in the physicochemical parameters. Most of the species of aquaculture interest have offspring whose size at birth is very small, thus requiring a particular rich food adapted to the size of the mouth (e.g. live preys which are very rich in nutritive reserves). The objective of this study was to set up a production system of live prey, daphnia for the feeding of fish fry. It consisted of culturing microalgae that were used to fed daphnia reared to fed the fry of the African catfish, Clarias gariepinus. The temperature and dissolved oxygen content in the culture and rearing media were monitored throughout the experiment. The results on species identification showed that the cultured microalgae and reared daphnia species are Microcystis sp and Daphnia magna, respectively. Analyses of algal biomass and daphnia abundance show an increase in these parameters with time. Temperature variations do not affect algal biomass, daphnia abundance and fry growth. On the other hand, a decrease in dissolved oxygen content below a certain threshold (6mg/l) leads to a decrease in algal biomass and daphnia abundance. Among the three feeding rates 1.5g, 3g, 4.5g (the equivalent of 0.5L, 1L, and 1.5L of microalgae, respectively) that were used to feed daphnia, the feeding rate of 3g of biomass corresponding to 1L is more adequate as it gave the best production. The comparison of the effects of the types of feed shows that live daphnia give better growths of C. gariepinus fry than the artificial feed, which may be due to their adequate size and nutrient richness. Thus, this study contributes to the establishment of live prey production systems to boost local aquaculture development and demonstrates the importance of daphnia for the feeding of early fish life stages.
Keywords: african catfish, Claria gariepinus, Daphnia magna, fly, live prey, Microcystis sp, production system
Faced with the declining catches due to the collapse of natural stocks, aquaculture is considered the main alternative to ensure an adequate supply of fishery products.1–5 Unlike traditional aquaculture, the new aquaculture aims to control not only the complete production cycle of the farmed species, but also the entire food chain leading to it.6,7 Its development will certainly make it possible to cover the important needs in animal proteins and would reduce the massive outflow of foreign currency. However, this development remains linked to the availability of food, which is a key factor for the success of any livestock.1,3,8
The importance of zooplankton for feeding fish larvae has already been proven by several studies.9–13 In addition to brine shrimp Artemia which is very expensive and not available in rural tropical areas, the use of live prey such as rotifers and water fleas (daphnia) which are less common may be easier and less expensive.8,14–16 In the search for opportunities to replace brine shrimp Artemia with local zoo-planktonic species, mastering simple production techniques for these organisms is a key issue in private fish farms. This is particularly the case in Senegal where there is often a problem of monitoring the survival of first larval stages of the fish in the farming units.17,18
Most Senegalese farms do not have the possibility to obtain all brine shrimp Artemia due to their high cost, which forces them to use artificial food that is sometimes larger than the size of the fish larvae's mouth. Aware of the difficulties faced by the aquaculture sector on the feeding of the first stages of development (larvae, fry), this study was carried out at the fish farm of Bel-Air in Dakar to set up a production system of live prey to meet their nutritional needs. Indeed, the farming of aquatic species, especially fish, is partly confronted with several problems related to the unavailability of appropriate food, adapted to the early stages of development. Almost all species of aquaculture interest have offspring whose size at birth does not exceed one centimeter.
To overcome this problem, we conducted an experiment that consisted in setting up a system for the production of live prey of daphnia (cladocerans). These have characteristics such as ease of production at low cost, efficiency in the regulation of phytoplankton, importance in the diet and adequate size for the larvae, which give them a huge nutritional potential for the early stages of development of fish species of aquaculture interest.19,20 Daphnia are arthropods that, together with other aquatic organisms, form the zooplankton.20 They are filter-feeders and derive their nutrient reserves from the food they consume, which are essentially microalgae.21,22 Daphnia are a very rich food source for many fish species of aquaculture interest, both for the early stages of development (larvae, fry) and for the advanced stages (juveniles, adults).23
The experiments carried out consisted in making cultures of microalgae that were used to feed daphnia reared to feed the fry of the African catfish, Clarias gariepinus. To this end, the C. gariepinus fry were divided into two batches, one of which was fed with live daphnia and the other with artificial food. This experiment was performed in duplicate, which allowed for a better evaluation of the effect of these two types of food on fry growth performances. In parallel to this main objective, this study has specific objectives which consist in isolating and identifying the species of microalgae and daphnia used, to assess the dynamics of predation of daphnia on microalgae, to determine the physico-chemical parameters for a good production of these species and to evaluate the effectiveness of daphnia on the feeding of fish larvae or fry.
Presentation of the study site: The experiments were carried out in the fish farming greenhouse of the Institute of Research for Development (IRD) of Bel-Air in Dakar, created within the framework of a partnership between Oceanographic Research Centre Dakar-Thiaroye (CRODT) and IRD. This facility includes different aquaculture installations distributed in different circuits used for many operations including broodstock conditioning, artificial fertilization and egg incubation, sexual inversion and fry rearing. These different facilities are powered by an air compressor, which is a source of oxygen for the fish. The facility also includes a microalgae and prey production system (Figure 1). The second stage of this system is used for the production of algae while the first stage is dedicated to the production of live prey (Figure 1). This system is also supplied with oxygen by the same air compressor.
Algae transfer: The harvesting of the algae was done in the large rearing tank of the Gaston Berger University fish farm (Saint-Louis). After sampling, these algae were put in 1L bottles and transported to the EPLS (hope of health) hatchery (Saint-Louis, Senegal) for species identification. Unfortunately, the identification tests at the hatchery did not allow us to determine the name of the strain. However, they did provide information on its size and shape. Further identification tests were carried out after the algae were transferred to the IRD in Bel-Air (Dakar), with the help of the technician responsible for the fish greenhouse.
Transfer of daphnia and growing: Daphnia were also collected in the EPLS hatchery. After harvesting, they were placed in oxygenated plastic bags and transported to the IRD greenhouse under optimal temperature conditions. Upon arrival, they were transferred to a 10L bottle and acclimated for 3 hours at room temperature. After acclimation, the daphnia were divided into two batches of 3L and 2L, respectively, and stored in two 10L bottles. They were then left for 30 minutes while they overcame the stress caused by handling. To avoid mortality due to stress and/or refusal to feed, only the first batch was fed the first day with 1L (3g) of microalgae. After observation the next day, it was found that the daphnia consumed the microalgae. Thus, the second batch (2L bottle) was fed with 1L of microalgae.
Isolation and identification of microalgae: The isolation consisted of collecting 5ml of algae from each 10L bottle using a syringe and placing them in 5ml plastic tubes. These tubes were then centrifuged at 10,000 rpm for 10 minutes with a Sigma centrifuge. After centrifugation, the supernatant was discarded and the substance deposited at the bottom of the tube consisting mainly of algae was kept and used for species identification. For this purpose, 50ml of the parental strain was taken to start the culture of the algae. This culture (50ml of parent strain) was then diluted by adding 500ml of water and 5g of NPK. This dilution was then exposed to light for a very fast development of the microalgae. After isolation of the microalgae, the strain was identified by microscopic observations with the most adequate magnification (10x40). The observed images were then photographed and compared to known classes of microalgae in order to identify the cultured algal strain.
Algae culture system: The algae culture system has been installed in the IRD greenhouse (Figure 1). It consists of 10L bottles containing the algae solution, which are installed on boards. These are connected to a pump by pipes to reduce the oxygen in the culture medium. The system has three levels, but only the two first were used (Figure 1). The first level was dedicated to the production of algae and the second to the culture of daphnia.
The parental strain of algae was grown in another part of the greenhouse for one month. Natural light was used throughout the culture and the parameters (temperature and dissolved oxygen) were in the required conditions. After isolation and identification of the parental strain, three bottles of microalgae culture (A1, A2 and A3) were prepared and used to feed daphnia. A1, A2 and A3 contained 1.5g, 3g, 4.5g of microalgae biomass corresponding to 0.5L, 1L, and 1.5L of microalgae, respectively.
Three 18L tanks named B1, B2 and B3 respectively were used for daphnia rearing, which was started by filling 5L of the daphnia containing solution in each tank. This resulted in the production of a large number of daphnia. The figure 1 illustrates the algae and daphnia production system.
Feeding rate: The feeding rate is the amount of food given to each tank of live daphnia. Since different feeding rates should be tested, three 5L culture tanks containing the same number of daphnia (5 individuals per 20 ml) were prepared. The daphnia in these three tanks (B1, B2, and B3) were d with 0.5L, 1L, and 1.5L, corresponding to 1.5g, 3g and 4.5g of microalgal biomass, respectively. It should be noted, however, that for B2, 3g of algal biomass was supplied from the 15th day of culture. From this day on (day 15), daphnia were fed three times a week (Monday, Wednesday and Friday) during the whole culture period. On the other hand, to maintain the culture of daphnia, the volume of water was reduced while maintaining the number of daphnia in each rearing medium and the feeding rate given for each tray (in number of litter). This was done by filtration and to avoid altering prey abundance. Very adequate mesh screens were used to prevent daphnia from passing through.
Biomass dynamics and physicochemical parameters: For the study of algal biomass, three samples of 100ml each were taken per week from each 10L bottle. After collection, the samples were brought to the laboratory to collect the biomass. Thus, each sample was first homogenized with a bubbler, before being filtered with a filter paper. Thus, the obtained biomass was dried in an oven. The incubation time in the oven to completely dry the biomass was 4 hours. The dried biomass was then weighed with a precision balance to obtain the weight in 100 ml. The evolution of the microalgae biomass as a function of time was then estimated. The physicochemical parameters (temperature and oxygen) were regularly recorded daily with a thermometer and an oximeter, respectively.
Fixation, identification and abundance of daphnia: Daphnia were fixed with formalin (37-38% formaldehyde solution) stabilized with PA-ACS methanol. The process involved taking 20ml of the solution from each 5L tank of the daphnia culture. Five drops of formalin were then added to each sample to facilitate fixation of the daphnia and maintain their size and shape. Identification of live prey was performed in the Saint-Louis EPLS hatchery using a microscope with magnification 10x40. Under the microscope, daphnia move very quickly and consume a lot of food (micro-algae). To confirm the species, a second microscopic identification test was performed at the IRD Bel-Air with the same magnification.
After fixing the daphnia, a few milliliters were poured onto a counting plate, which was then placed on the microscope to visualize the species and count the number of individuals. A count was carried out 3 times a week with a regular control of the physicochemical parameters every morning. The use of the alga as food for the daphnia and good culture conditions contribute to the increase of their size, which should evolve with time. Daphnia size was estimated at each count from the photos (x100 magnification) that were taken.
Effects of daphnia on the growth performances of fish fry: To evaluate the effects of daphnia on the growth performance of African catfish C. gariepinus fry, a comparative analysis was conducted over a period of 7 days (from hatching to weaning) with regular monitoring of physicochemical parameters. It consisted in feeding the fry with natural food (daphnia) and artificial food and to compare the effects of these two types of food on their growth performance. The experiment was conducted on 4 tanks (D1, D2, D3 and D4) each containing 100 fry of African catfish. Two batches of fry (duplicate: D1 and D2) were fed with the daphnia (natural food) and the other two (duplicate: D3 and D4) with the artificial food. The initial weight of the fry was calculated for each tank. At the end of the experiment, the final weight was calculated and the survival rate was determined. The survival rate was calculated by dividing the number of live fry after a given time interval by the initial number.
Physicochemical parameters: The temperatures of the three bottles of microalgae culture (A1, A2 and A3), daphnia rearing tanks (B1, B2 and B3) and breeding tanks of African catfish fry (D1, D2, D3 and D4) were daily measured with a thermometer. The dissolved oxygen levels were also daily measured in the microalgae culture bottles, daphnia rearing tanks and breeding tanks of African catfish fry.
Data processing: All collected data were stored in an Excel file and processed with XLSTAT software. This program was used to test the normality of the distribution with the Shapiro-Wilk test but also the homogeneity of the variances with the Levene test. The distribution was normal but the variances were not homogeneous. Therefore, the Kruskal Wallis test was applied to test the significance of algae biomass and the daphnia abundance. Multiple linear regression analyses were performed to show correlations between oxygen, temperature, algal biomass and daphnia abundance. For the same data, analyses of variance were also performed to assess significance. Student's t test was also used to compare the mean growth rates of fry fed with daphnia and artificial feed. The threshold of significance retained is 5%.
Microalgae isolated and identified: The genus of microalgae determined after the isolation and identification is Microcystis sp. The genus Microcystis is characterized by small cells (a few micrometers in diameter) with gas-filled vesicles (no individual sheath). The cells are usually organized in colonies that are bound by thick mucilage composed of complex polysaccharides (Figure 2). Figure 2A is a photo of the species taken at high magnification while Figure 2B is a photo that was taken at low magnification.
Daphnia fixed and identified: The species of daphnia identified after isolation and fixation is Daphnia magna (Figure 2C). This species is characterized by its small size which does not exceed 6 mm and its subdivision into two parts: the head and the body. The head has a compound eye, a mouth while the body is covered by a transparent shell that reveals the internal composition of the species. The body is extended by a caudal spine. In addition, photos of daphnia taken during the identification of the species showed black colored individuals (Figure 2D). These black individuals are ephippia also called hard eggs.
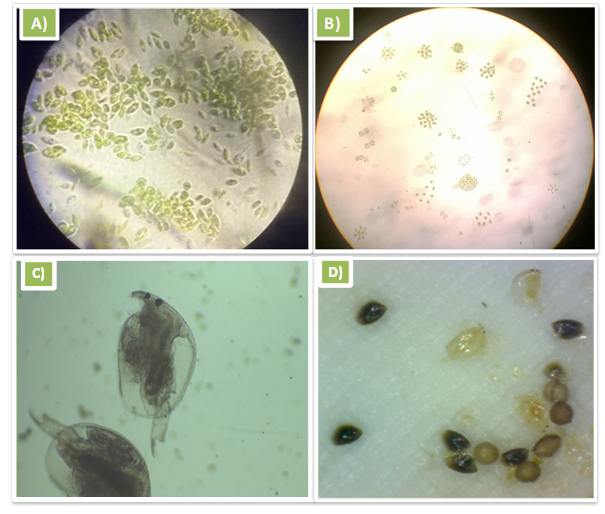
Figure 2 Images of Microcystis sp and Daphnia magna taken with a light microscope with different magnifications (A: x100, B: x40 and C: x100, respectively). The image D is a photo of duration eggs or ephippia of Daphnia manga taken in August 2018.
Algae biomass: The evolution of algal biomass shows an increase with time in all three bottles (A1, A2 and A3) (Figure 3A). The curves have a similar profile in the three bottles but there is a decrease in algal biomass in A1 at the 21st day, which is not observed in A2 and A3. Biomass in all three bottles reached 3g at day 15 and continues to grow until 27th day with an average biomass of 3.75g. The maximum biomass was reached in A1, A2, and A3 at 30th, 24th, and 27th days, respectively.
Abundance of daphnia: Figure 3B below shows a much more marked evolution of the daphnia abundance in B2 with a maximum growth (121 individuals) from the 15th day. For B1, an increase in daphnia abundance is also observed with time, but much slower (maximum of 60 individuals reached on day 24) than that of B2 (Figure B). For B3, daphnia abundance increases from 1st to 12th day and decreases from 12th to 21st. Then, it increases from 21st to 27th day. Maximum daphnia abundance in the rearing tanks (58 individuals) was reached from 12th day onwards. Pairwise comparisons of mean daphnia abundance showed no significant difference between groups B1, B2 and B3 (Kruskal-Wallis test, p = 0.179) although it decreases from day 12th to 21st for B3 and from day 18th to 21st for B2 while it increased for B1 during this period.
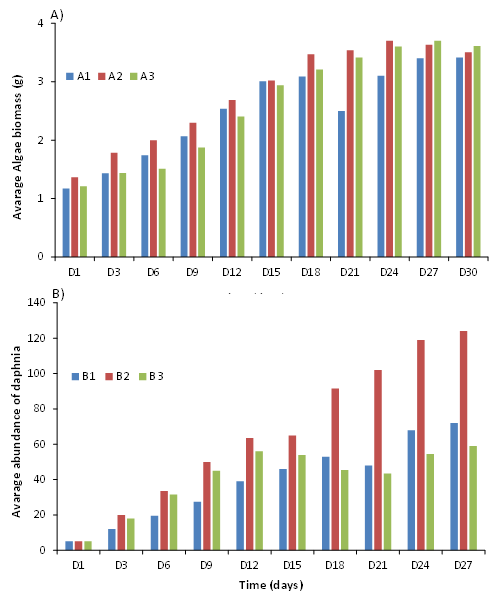
Figure 3 Changes in algal biomass of the three 10L bottles (A) and average daphnia abundance in the three tanks (B) as a function of time.
Physicochemical parameters in culture media: The physicochemical parameters (temperature and dissolved oxygen) measured in the algal culture media and in the daphnia and African catfish rearing tanks show variations with time. The temperature in the algal culture media (bottles A1, A2 and A3) varied between 27.6°C and 29.1°C. The temperature variations are more pronounced in bottles A1 and A2 compared to bottle A3 where the lowest variations were recorded (Figure 4A). There is no significant difference in temperature in the culture media between the beginning and end of the experiment (Kruskal Wallis; P> 0.05).
The temperature in the daphnia rearing tanks (B1, B2 and B3) varied between 26°C and 28.3°C. The highest temperatures in tanks B1, B2 and B3 were reached on days 2, 4 and 11, respectively (Figure 4B). The temperature in the breeding tanks of the reared African catfish fry did not show significant differences between replicates D1/D2 and D3/D4 (Figure 4C). In all tanks, water temperature decreased with time until 4th day when it reached its minimum (24.2°C). It then increases slightly in all tanks until 7th day (Figure 4C). The average temperature results show that in the tanks, it was slightly higher in the evening than in the morning (Figure 4C). There was no significant difference in the average temperature between the rearing tanks (Kruskal Wallis; P> 0.05).
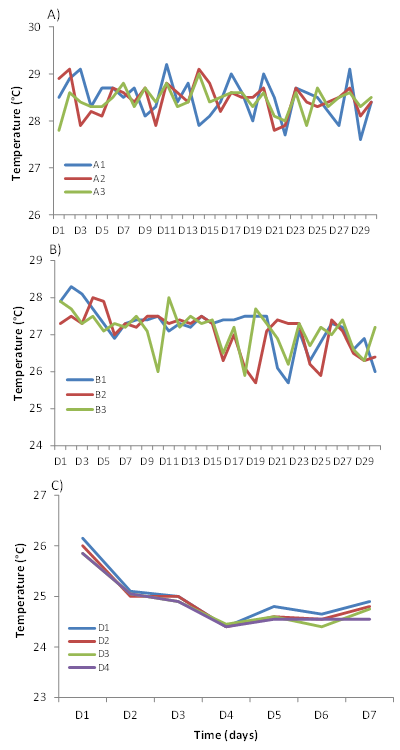
Figure 4 Variations with time of the water temperature of Microcystis sp (A) and Daphnia magna (B) culture media and Claria gariepinus rearing medium (C).
Dissolved oxygen: Oxygen levels in the algal growth and reproduction media also show variation with time. The oxygen content in the three bottles (A1, A2, A3) varied between 5.2 and 9 mg/l (Figure 5A). It should be noted, however, that the oxygen content dropped considerably on day 21 in bottle A1 where it reached the minimum value (1.7 mg/l). No significant difference in average oxygen content was observed in the three bottles between the beginning and end of the experiment (Kruskal Wallis; P > 0.05). The oxygen content was not very significant between A1, A2, and A3 (Kruskal Wallis; P > 0.05).
Daily measurements of oxygen content in the daphnia tanks (B1, B2, and B3) show that it varied between 7.3 and 3.8 mg/l (Figure 5B). It should be noted, however, that oxygen levels fell below this 3.8mg/l value on 20th and 21st days in B1 and B3, respectively. Oxygen levels were higher at the beginning than at the end of the experiment (Kruskal Wallis; P < 0.05). Comparison of oxygen levels between tanks showed no significant differences between B1, B2 and B3.
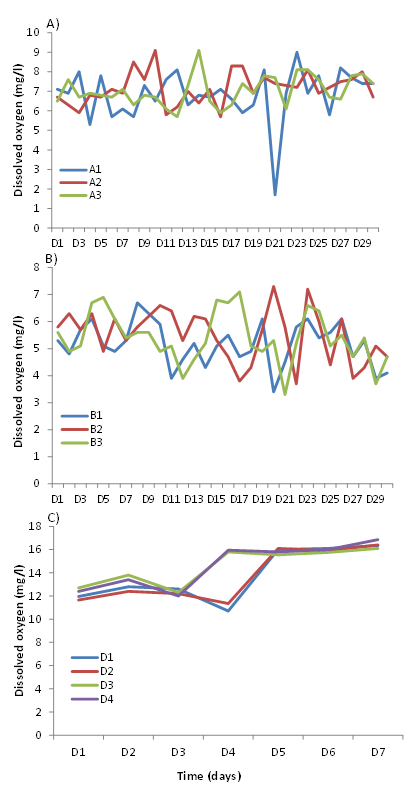
Figure 5 Variations with time of dissolved oxygen levels in the Microcystis sp (A) and Daphnia magna (B) culture media and in Claria gariepinus rearing medium (C).
Comparison of average oxygen levels in the African catfish fry rearing tanks shows slight increases as a function of time with higher levels at the end of the experiment (16.8 vs. 10.1 mg/l) (Figure 5C). It also shows that morning oxygen levels are slightly higher than evening levels. However, oxygen levels dropped in replicates D1/D2 on day 4 while they continued to increase in replicates D3/D4 (Figure 5C).
Relationship between oxygen and algal biomass: There is no significant correlation between oxygen content and algal biomass in all culture bottles (Table 1). However, algal biomass increases significantly in all three culture bottles when oxygen content is greater than or equal to 6 mg/l (Figure 6). A decrease in oxygen content below this value is associated with a decrease in algal biomass (Figure 6A–C). This was particularly seen in A1 where a decrease in oxygen content to 1.7mg/l on day 21 caused a considerable decrease in algal biomass from 3.1 to 2.5g (Figure 6A). Indeed, while an increase in oxygen content is always associated with an increase in algal biomass, its decrease is not always companied by a decrease in algal biomass (Figure 6). It appears that oxygen levels must fall below a certain threshold value for algal biomass to be affected. This was found in bottle A1 where a drop in oxygen level on day 21 is associated with a considerable decrease in biomass (Figure 6A).
Bottle |
Equation |
R² |
P-value |
A1 |
Y= -0.02x+ 2.60 |
0.01 |
> 0.05 |
A2 |
Y=0.15x + 1.89 |
0.38 |
> 0.05 |
A3 |
Y = 0.14X + 1.76 |
0.24 |
> 0.05 |
Table 1 Relationship between oxygen content and biomass of the alga Microcystis sp
Relationship between oxygen and daphnia abundance: The average oxygen level in the daphnia culture tanks (B1, B2, and B3) ranged from 4.4 to 6mg/l (Figure 6). Daphnia abundance was not significantly correlated with oxygen content (Table 2). Indeed, an increase in oxygen level is not always accompanied by an increase in abundance (Table 2, Figure 6). However, the results show relationships between these two parameters in B2, B2 and B3 (Figure 6). Indeed, the decrease in oxygen level in B3 from day 9 to 12 is followed by a decrease in daphnia abundance from day 12 to day 21 (Figure 6C). Similarly, the decrease in oxygen level between days 18 and 21 is associated with a decrease in daphnia abundance in B1 and B3 (Figure 6A, C). In contrast, changes in oxygen level in B2 are not accompanied by changes in Daphnia abundance (Figure 6C). In B2, daphnia abundance increased with time from 1st to day 27th day.
Tanks |
Equation |
R² |
P-value |
B1 |
Y = -3.09X+56.03 |
0.17 |
> 0.05 |
B2 |
Y = -4.25X+90.73 |
0.09 |
> 0.05 |
B3 |
Y = -0.41X+43.47 |
0.005 |
> 0.05 |
Table 2 Relationship between oxygen content and daphnia abundance
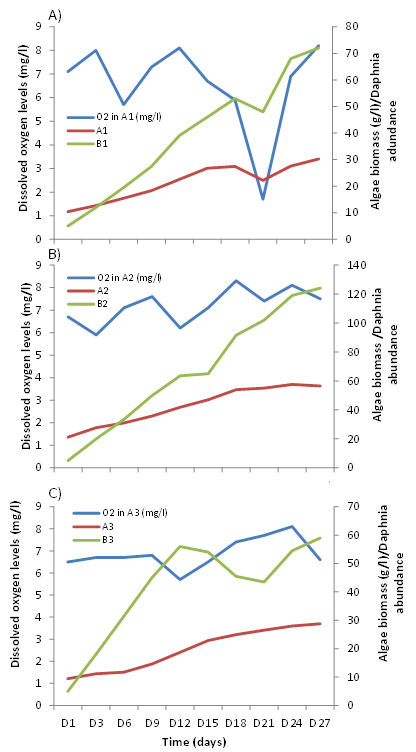
Figure 6 Relationship between dissolved oxygen levels in the algal culture medium, algal biomass and daphnia abundance.
Relationship between algal biomass and daphnia abundance: The analysis of algal biomass and daphnia abundance shows a significant correlation between these two parameters (Table 3, Figure 7A). Indeed, in all three tanks, an increase in daphnia abundance is observed when the algal biomass increases. And a decrease in algal biomass leads to a decrease in daphnia abundance, which was particularly observed in tank B1 fed by bottle A1 (Figure 7A). The average abundance of daphnia and algal biomass as a function of time showed similar patterns (Figure 7B). Indeed, both daphnia abundance and algal biomass increase with time from the 1st to 18th day. On day 21, a decrease in algal biomass is observed, which is associated with a decrease in daphnia abundance (Figure 7B). Both parameters then increase with time from day 24 onwards.
Bottle/Tank |
Equation |
R² |
P-value |
A1/B1 |
Y=7.33x-1.3 |
0.95 |
< 0.05 |
A2/B2 |
Y=13.47x-6.73 |
0.98 |
< 0.05 |
A3/B3 |
Y=4.84x+14.5 |
0.68 |
< 0.05 |
Table 3 Relationship between algal biomass and daphnia abundance

Figure 7 Relationships between dissolved oxygen levels in the daphnia culture tanks their daphnia abundance (A) and between the average of algae biomass and daphnia abundance (B).
Effect of daphnia and artificial food on larval growth: Comparative analysis of growth performance shows that the best growths were obtained in fry fed with live prey (daphnia) and the lowest in those fed with artificial food (Student-t tests; P < 0.05 (Figure 8A). Indeed, fry from the breeding tanks D3 and D4 (fed with daphnia food) have an average weight of 7g at the end of the experiment while those from tanks D1 and D2 (replicates fed with artificial food) have a weight of 3g (Figure 8A). The size at the end of the experiment shows that the fry fed with the natural or live prey (daphnia) were about 01cm long, which is twice the size of those fed with the artificial food (Figure 8B).
The food chain refers to a sequence of living organisms at different trophic levels in which each eats organisms at a lower trophic level to meet its energy needs.24 It also defines the relationship between microalgae, daphnia and fish larvae. This relationship is direct when some species consume the microalgae without passing through the Daphnia trophic level, while it is indirect when fish use daphnia as a food resource. Daphnia feed on microalgae (first link in the food chain) and, like other cladocerans, they play key roles in freshwater ecosystems25 because of their numerous and diverse predators, namely fish (juveniles and adults). Indeed, daphnia are a very rich food source for many fish species of aquaculture interest, both in the early (larvae, fry) and late (juveniles, adults) stages of their development. The objective of this study was, therefore, to establish a daphnia production system that could be used as live prey for fish fry in rearing systems, especially for young stages that are very demanding in terms of food quality. The experiments carried out to achieve this objective consisted in making cultures of microalgae that were used to feed daphnia, which in turn were raised to feed African catfish fry. The results obtained showed that daphnia can boost the growth of C. gariepinus and could thus contribute to optimize the production yields of this species but also that of other fish species exploited in aquaculture.
Identification of the species: The identification results show that the identified and cultured species is characterized by small cells (a few micrometers in diameter), with gas-filled vesicles, indicating that it is Microcystis sp.26 They also show that the daphnia species that was identified has characteristics such as a large size for orientation, which are similar to those described by Amoros27 for Daphnia magna. According to Haroun Haroun,8 the size of D. magna varies between 3 and 5 mm and its body is subdivided into two parts which are the head that bears a compound and mobile eye and the body that is covered by a transparent carapace.27 Its nervous system is characterized by the presence of a cerebral ganglion located between the eye and the beginning of the digestive tract.27
The chitinous shell of these eggs whose appearance is due to unfavorable conditions has earned them the name ephippia, and correspond to individuals of black color whose presence is due to the conditions of the unfavorable environment (lack of food, lack of oxygen etc...). In such conditions, the eggs produced by daphnia do not hatch. They are then called eggs of duration. Observations in this study show that the pouch in which the eggs are housed is saddle-shaped and surrounds the back of the female daphnia, which is in agreement with the results obtained by Boehler.28 The chitinous shell of these eggs whose appearance is due to unfavorable conditions has earned them the name ephippia. It should be noted, however, that if conditions return to normal, these resistance eggs hatch but give rise to a population that is genetically different from the parental daphnia.29
Biomass and abundance of algae: The conditions of algal and daphnia culture media can influence their biomass and abundance, respectively.30,31 Indeed, the physicochemical parameters of these media should be taken into account for a good growth and production of Microcystis sp or D. magna. These must be favorable to ensure an optimal biomass of microalgae and abundance of daphnia. Thus, for the survival of Microcystis sp, the water temperature of the culture medium must be between 20 and 28°C. The optimal temperature for the development of this species is 25°C. In addition to the temperature, this species of microalgae is very demanding in oxygen. Indeed, the oxygen content of the culture medium must be between 5 and 7mg/l for a good survival of the species. For daphnia, the minimum temperature required for survival is between 25 and 25.7°C.32 The maximum temperature is 30°C and the optimum temperature is between 18 and 22°C. Regarding the oxygen content, the normal level for good survival of daphnia is about 7 mg/l.
The results of this study show a good production of Microcystis sp in the culture medium where the temperature is between 27.6°C and 29.1°C. These results suggest that this temperature range is favorable for the multiplication and growth of the species. For D. magna, a time-dependent increase in abundance was observed in a temperature range between 26 and 28.3°C, suggesting that these values would also be favorable for the survival and growth of the species. However, for both species, the temperature range in which they were maintained is higher than the optimal temperature for their survival and growth, making it difficult to compare our results with those of previous studies.33–38 Indeed, it is impossible to conclude on the optimal temperature for growth of the two species because those proposed by previous studies were not tested in this study.34,39–41 Further studies on the effect of different temperatures on the growth of these two species are needed to determine the optimal temperature for their survival and growth.
The results of this study show that the biomass of Microciystis sp and D. magna in the culture media increases when the oxygen content is greater than or equal to 6mg/l. This value is close to the threshold (7mg/l) that has been proposed as normal for a good survival of these species.42,43 A decrease in the oxygen content of the culture medium below this threshold would result in a decrease in the biomass of the alga produced, as was observed in culture medium A1. Indeed, in this bottle, a decrease in oxygen to 1.7mg/l on day 21 resulted in a significant decrease in algal biomass from 3.1g to 2.5g. These results indicate that dissolved oxygen deficiency can strongly influence microalgal production.44,45
The abundance of D. magna in the rearing tanks increases when the oxygen content is greater than or equal to 6mg/l (near threshold value 7mg/l). It has been shown that the amount of eggs laid by a daphnia depends on the dissolved oxygen content of the culture medium.46 The results of this study show that daphnia abundance appears to be affected by dissolved oxygen content when the dissolved oxygen content is less than 5mg/l, in agreement with Haroun8 who observed a decrease in daphnia abundance in culture media when dissolved oxygen is deficient. The results also showed a significant positivity of daphnia abundance with microalgal biomass. Indeed, for all three feeding rates tested, daphnia abundance increased when microalgal biomass increased, indicating that microalgae affected daphnia growth. Consistent with this interpretation, a significant decrease in microalgal production was noted in A1 where a decrease in oxygen content was noted on day 21. This decrease in microalgal biomass due to a dissolved oxygen deficit resulted in a decrease in daphnia abundance. Since the latter serve as food for the daphnia, an increase in their production leads to an increase in daphnia abundance.
Differences in daphnia abundance were noted depending on the rate of microalgae feeding rate that was used to feed the daphnia in the rearing tanks. Among the three concentrations of microalgae used to feed daphnia, the feeding rate of 1L corresponding to 3g of biomass gave better production. Indeed, in order to reproduce and develop properly, daphnia need a sufficient amount of food available to their palatability, which seems to be the case in tank B2 where a clear and significant increase in daphnia abundance with time was noted. The feeding rate of 1L (3g biomass) of algae resulted in a very rapid multiplication of daphnia, which is in agreement with the results of Lynch and Ennis47 and Cowgill al.48 who argue that the quantity and quality of food play a determining role in reproduction and clearly influence the population dynamics of daphnia. In tank B1, where the feeding rate was 1.5 g of microalgal biomass (corresponding to 0.5L), there was a slight change in daphnia abundance, probably due to insufficient food.47 Although daphnia require sufficient food for multiplication and growth, saturation of food in the medium can lead to an accumulation of food at the bottom of the tank. This phenomenon makes the medium loaded with microalgae because not all the food is consumed. This could be the case for tank B3 where the highest feeding rate 4.5g of microalgal biomass corresponding to 1.5L was used. This high concentration of microalgae causes a saturation of the culture medium (B3), which may lead to stress, and consequently a slowing down of their multiplication and growth in individuals. This could lead to a decrease in daphnia production. During the experiment, we noticed that a large amount of food was retained at the bottom of tank B3 compared to tanks B1 and B2. Indeed, the excess of microalgae overloads the culture medium of the daphnia which leads to uneaten food and consequently to a mortality rate of the species.
Importance of daphnia use for C. gariepinus fry: Comparison of the effects of the two types of food on the growth of African catfish fry showed significant differences in body weight of individuals. Fry fed with the natural food (daphnia) had a weight and size that was more than double that of those fed with the artificial food. This difference in size is probably not due to differences in the physico-chemical parameters such as temperature and dissolved oxygen content. Indeed, the temperature in the rearing tanks of fry fed with live daphnia was not significantly different from that in the rearing tanks where fry was fed with artificial food. In this study, the temperature of the fry rearing tanks varied between 24.2 and 26.8°C. This temperature range would be favorable for the species because according to49 the temperature range favorable of C. gariepinus is between 20 and 30°C with an optimum of 27°C for juveniles and 25°C for adults. Similarly, Hogendoorn et al.50 observed optimal growth of juveniles (0.5g) between 27.5 and 32.5°C with normal growth at 25°C. These differences in body weight batches fed with the two types of food would not be due to differences in dissolved oxygen content in the tanks. Indeed, the dissolved oxygen content did not show significant differences between the batches of fry fed with artificial feed and the batches fed with live daphnia except on day 4 when there was a decrease in oxygen level in the tanks of the batches fed with artificial feed (D1, D2). It should be noted, however, that despite this decrease, the oxygen level in all tanks did not represent a limiting factor for growth as it remained above the threshold of 3mmg/l which is the concentration required for good growth of individuals.49
The differences in body weight observed in this study show that live daphnia are more effective than artificial food for the growth of C. gariepinus fry. These results are consistent with those of Haroun8 who showed that larvae fed with the daphnia have a greater body weight than those fed the artificial food. It has also been shown that C. gariepinus fry clearly prefer live daphnia, due to the fact that the latter are much easier to ingest.51 This difference in weight can be explained by the fact that live prey in general and daphnia in particular, have a higher nutritional potential (lipids, proteins, carbohydrates, vitamins, etc.) than artificial food.
Aquaculture in Senegal often encounters problems especially during the early stages of development (larval stages, fry, and juveniles). The main reason for this, according to several studies, is the unavailability of an efficient feed adapted to the size of the larvae and fry. Aware of this situation, we have tried to develop a production system of microalgae and live prey. The microalga Microcystis sp was used to feed live daphnia (D. magna) in comparison to an artificial diet. Evaluation of the effect of three different feeding rates showed that the adequate and recommended amount is about 3g of microalgae biomass (5L) of daphnia from the 15th day of culture. The food should depend on the palatability and color of the prey environment. For example, to feed fish fry (C. gariepinus), daphnia are the most appropriate compared to artificial food, because they are smaller than the mouth of fish larvae. Indeed, daphnia, whose rearing depends on biotic and abiotic factors, are an important link in the food chain. They proliferate rapidly, which makes them of great interest for larval rearing. They can be used in several applications.
This study contributes to the establishment of methods for the production of live prey and demonstrates their importance for the feeding of early life stages of fish. However, it would be important to conduct further studies to optimize their production by evaluating the optimal physicochemical parameters (temperature, dissolved oxygen, pH etc...) for their growth and reproduction. It would also be interesting to try to integrate the production of live prey in the fish farming systems for the feeding of the early development stages of fish. These can maximize growth performance and reduce larval mortality rates. However, ideally, live prey culture should be started two weeks prior to the hatching of larval fish to ensure sufficient food before they are born. In addition to daphnia, other organisms such as rotifers and earthworms can be tested as live prey for the early stages of fish development.
The project was funded by the authors with the support of Senegalese government.
Not applicable.
Not applicable.
All data related to this manuscript are presented in the main text, figures and tables.
The authors thank their Institutes and the Senegalese government for supporting this study.
The authors declared no conflict of interest.

©2022 Tine, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.