Journal of
eISSN: 2378-3184


Research Article Volume 4 Issue 6
Department of Zoology, Kurukshetra University, India
Correspondence: Anita Bhatnagar, Department of Zoology, Kurukshetra University, India, Tel 0091-01744-238410; +91 09896634580
Received: November 01, 2016 | Published: December 28, 2016
Citation: DOI: 10.15406/jamb.2016.04.00102
The effects of supplementing diets with levamisole on growth, immune response and resistance against pathogenic Aeromonas hydrophila were determined. Fish were assigned to three treatments with different level of levamisole (125, 250 and 500 mg kg¯¹ diet) and the control diet was prepared without levamisole. Cirrhinus mrigala were fed at 4% of their body mass for 60 days. Growth performance, enzyme activities and immunological parameters were analyzed among groups. The total erythrocyte count, total leucocyte count, phagocytic activity and NBT reduction were significantly (P<0.05) enhanced in levamisole treated group when compared to control. The relative percentage survival, A. hydrophila post-challenge was significantly higher in fishes fed on 250 mg kg¯¹ of levamisole supplemented diet. The same dose also resulted in significantly high specific growth rate (SGR), feed conversion ratio (FCR), apparent protein digestibility (APD) and elevated intestinal enzyme activities compared to other groups. Thus, administration of 250 mg of levamisole kg¯¹ diet is recommended for enhancement of immunity, growth and survival of C. mrigala hence acting as a potent immunostimulant agent
Keywords:Aeromonas hydrophila, Mrigala, Enzyme activities, Growth performance, Immunological parameters
PR, Phagocytic Ratio; PI, Phagocytic Index; SGR, Specific Growth Rate; PER, Protein Efficiency Ratio; GCE, Gross Conversion Efficiency; APD, Apparent Protein Digestibility; FCR, Feed Conversion Ratio
With increasing trend of searching different harmonic measures for preventing fish diseases, imunnostimulants are gaining popularity as an attractive and promising alternative to chemotherapeuants in aquaculture practices. Immunostimulants comprise a group of biological or synthetic substances known to stimulate the non-specific immune mechanisms of host on their own or specific immune mechanism when conjugated with some antigen. It is well known that fish rely more profoundly on nonspecific defense mechanism than mammals.1 Immunostimulants elicit an effective and profound immune response to those infectious agents such as viruses, bacteria, fungi, and parasites without risks of toxicity, carcinogenicity or tissue residues.2 Hence, immunostimulants as dietary additives appears to be a valuable possibility in enhancing disease resistance of fishes.
Levamisole- a synthetic phenylimidazolthiazole, is an antihelminthic drug which has been widely in use for the treatment of nematode infections in man and animals. Incidental observations implied that upon treatment with levamisole a state of enhanced resistance to various kinds of infection was achieved. The ability of this agent to positively regulate the immune response of both healthy3 and immunocompromised individuals is well documented.4-6 It was the first drug reported to enhance various aspects of cellular immunity among healthy laboratory animals.3 In fish, levamisole has been used in various studies with the intention of enhancing the nonspecific immune response7-12 or as an adjuvant with a vaccine.13,14 Its action has been reported in several studies as: modulation of T-cell function, cytotoxic activity of leucocytes, enhanced phagocytosis and respiratory burst and activation of macrophage-activating factor. Besides imparting immunity, it’s capability of enhancing resistance to pathogenic bacteria in several fish species have been reported.10 Alongside the immunostimulating influence and potential use in fish health management, the role of levamisole role in growth promotion and digestibility is also well documented.15 Hence, further expedition on the possible effect of dietary administration of levamisole on fish growth; digestibility, immunity and resistance against pathogens need to be determined. Present study aims to assess the growth, biochemical parameters, water quality, immune response and disease resistance of Cirrhinus mrigala (average weight of 1.68±0.04 g) fed on diet containing different concentration of levamisole under laboratory conditions.
Fish and rearing conditions
The experimental Cirrhinus mrigala were collected from a local fish farm (Jasbeer fish seed farm, Adon, Kurukshetra, India). The fish were stocked in a re-circulating water tubs and acclimatized to the ambient laboratory condition. The fish were fed with basal diet for 10 days, prior to starting the experiment.
Experimental diets
A basic diet with 40% protein and about 9.10% fat was prepared that contained ground nut oil cake 650, rice bran 42, hydrothermically processed soybean 266, wheat flour 32, mineral mixture 10 (in kg g-1) which was considered the control diet. Levamisole was procured from Rowan Bioceuticals Private Limited, New Delhi. In processed soybean based feed, powdered levamisole was added @ 125 mg for diet L1, 250 mg for treatment L2 and 500 mg for treatment L3 (See Table 1 for proximate composition of ingredients). Diet with no levamisole served as the control diet (LC). All dietary ingredients were mixed thoroughly by adding warm distilled water and homogeneous dough was prepared. The resulting dough was passed through a mincer to produce feed pellets of 0.5 mm in diameter. Then, pellets were first air dried and packed in airtight containers. The diets were kept in a refrigerator at 4ºC until use. All these diets were isocaloric and isoproteic with approximately 40% proteins (Table 2).
|
Ingredients |
Crude protein (%) |
Crude fat (%) |
Crude fiber (%) |
Ash (%) |
Moisture(%) |
NFE (%) |
Gross energy (kJg-1) |
|
Soybean |
43.53±0.19 |
15.86±0.18 |
6.51±0.02 |
6.83±0.02 |
6.06±0.53 |
23.22±2.34 |
19.54±2.78 |
|
Rice Bran |
12.18±0.23 |
10.17±0.01 |
16.64±0.04 |
10.48±0.09 |
8.68±0.37 |
41.65±3.66 |
14.04±1.02 |
|
Wheat Flour |
11.28±0.23 |
4.46±0.03 |
10.83±0.03 |
7.76±0.8 |
8.48±0.29 |
57.19±5.17 |
14.23±1.16 |
|
Groundnut Oil Cake |
42.09±0.23 |
5.68±0.04 |
8.29±0.04 |
5.97±0.03 |
6.72±0.27 |
31.25±3.25 |
16.33±2.13 |
Table 1 Proximate composition of different ingredients used in experimental feeds for Cirrhinus mrigala.|
All values are Mean ± S.E of mean
|
Proximate analysis
|
Dietary treatments |
|||
|
LC (control) |
L1 (125 mg) |
L2 (250 mg) |
L3 (500 mg) |
|
|
Crude protein ( % ) |
39.85±1.36A |
39.00±1.20A |
39.40±1.78A |
38.80±1.95A |
|
Crude fat ( % ) |
9.10±0.26B |
9.31±0.36A |
9.35±0.05A |
9.28±0.13A |
|
Crude fiber (% ) |
6.23±0.06A |
6.05±0.18B |
6.26±0.21A |
6.20±0.15A |
|
Total ash ( % ) |
6.60±0.39C |
6.80±0.33A |
6.76±0.13B |
6.83±0.11A |
|
Moisture ( % ) |
7.41±0.20A |
7.35±0.5B |
7.40±0.66A |
7.46±0.29A |
|
Nitrogen free extract (% ) |
29.30±1.42B |
31.40±1.74A |
30.80±2.69AB |
31.40±1.79A |
|
Gross energy (kJ g-1) |
17.93±0.09A |
18.30±0.12A |
18.29±0.06A |
18.23±0.20A |
|
Feed phosphorus (%) |
1.34±0.07B |
1.33±0.03B |
1.38±0.09A |
1.42±0.07A |
Table 2 The composition (% dry weight basis) of experimental diets
Means with different letters in the same row are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
Experimental design
All dietary treatment groups were performed with triplicate of each. The experiment was conducted under laboratory conditions (25±1°C) in plastic tubs (30 L capacity). Each tub was filled with de-chlorinated tap water and then stocked with 20 advanced fish fry with average body weight of 1.68±0.04 g. All groups of fish were fed daily at 4% Body Weight (BW) in 2 instalments at 08:00 and 16:30 hours for 60 days with constant aeration and daily two-thirds water exchange. Individual weights of fish were recorded at the beginning and end of experiment and at every 15th day of interval with the help of top pan balance. Length of fish was measured using a simple centimeter scale. Initial and final fish samples were processed for proximate analysis following Association of Official Analytical Chemists.16
Proximate analysis of experimental diets (Table 1) and fish carcass (initial and final) was done following the standard methods of the AOAC.16 Chromic oxide levels in the diets as well as in the faecal samples were estimated spectrophotometrically.17 The intact pellets were picked up by tube dropper or siphon pipe and dried in oven in a petridish.
Apparent protein digestibility (APD) of the diets was calculated according to the methods of Cho et al.18 It was determined by an indirect method, using chromic oxide as a dietary inert external marker. As a dietary inert substance, chromic oxide (Cr2O3) is excreted without digestion or loss. Chromic oxide was incorporated into the diet and faecal samples were collected after a minimum period of feeding, normally 14 days. 100 mg of faecal samples and diet were assayed for Cr2O3 and the percentage dry matter digestibility was calculated according to Cho et al.18 Live weight gain (g), percent weight gain (PWG), specific growth rate (SGR), feed consumption per day in percentage of body weight, feed conversion ratio (FCR), gross conversion efficiency (GCE), and protein efficiency ratio (PER) were calculated using standard method.19 At the termination of experiment, from each treatment, six fish were randomly sampled and kept on ice to remove the intestines which were processed for the determination of enzyme activity of protease,20 amylase,21 and cellulose.22
During the experiment, the water samples from all the tubs were collected fortnightly and temperature, dissolved oxygen (DO), pH, electrical conductivity, calcium, chlorides and total alkalinity were measured following American Public Health Association23 to investigate the influence of supplemented feeds on quality of holding water. At the end of feeding trials, water samples from each tub were collected at two- hour intervals for the estimation of excretory levels of total ammonia (N-NH4+) and reactive orthophosphate following the APHA,23 and calculated following Sumagaysay-Chavoso.24
Collection of blood Samples
Blood samples were collected from caudal vein. Blood samples of five fishes were pooled for analysis.. EDTA (1 mg EDTA ml-1) was used as anticoagulant in blood.
Hematological measures
The blood samples were used for the estimation of total erythrocyte and leukocyte count with the help of using a Neubaur’s counting haemocytometer following the methods given by Dacie & Lewis.25
Non-specific immune response
Phagocytic assay and Phagocytic index: For phagocytic assay cells of freshly grown pathogenic bacteria Aeromonas hydrophila in 0.1 ml of PBS were added to 0.1 ml of blood sample (pooled samples of blood of five fishes mixed with EDTA as anticoagulant) of fishes of each treatment in sterile microplate. Blood was then incubated for 30 min at 25°C after thorough mixing in the well. The plate was removed and fifty microlitres of each suspension was transferred on glass slides to make smears. After air drying, smear were fixed in 95% ethanol, re-dried and stained with May Grunwald Giemsa. The phagocytic cells and phagocytosed bacteria were enumerated. Phagocytic ratio (PR) and phagocytic index (PI) were determined by enumerating 100 phagocytes per slide under a microscope. The average of three slides was calculated depending on the formula which is given below.
Phagocytic ratio (PR; i.e. percentage of cell with engulfed bacteria)=(No. of phagocytic cells with engulfed bacteria/No. of phagocytic cells) × 100.
Phagocytic index (PI; i.e. number of engulfed bacteria per cell)=No. of engulfed bacteria/No. of phagocytic cells.
Nitroblue tetrazolium (NBT) assay
The oxygen radical production by phagocytes in blood during respiratory burst activity was measured through nitroblue tetrazolium (NBT) assay as described by Anderson & Siwicki.26 Briefly, 0.1 ml of EDTA mixed blood from each treatment group was taken in Eppendorf to which 0.1 ml of 0.2% NBT solution was added. The mixture was incubated for 30 minutes at 25°C. From the suspension, 50 µl was taken, added to 1.0 ml N, N-dimethyl formamide in a glass tube and centrifuged at 3000 g for 5 minutes. The optical density (OD) of the supernatant was measured at 540 nm in the spectrophotometer.
Challenge test
After feeding for 60 days, 10 fishes from each treatment were challenged with Aeromonas hydrohila which has been cultured and maintained in the selective medium. Fishes in all replicates immersed in a suspension of A. hydrophila ~ 105 CFU ml-1 followed by a second immersion ~107 CFU ml-1 after 7 days.27 Per cent survival was measured for 10 days based on observation that mortality reached its plateau after 1 week28 and relative percentage survival was calculated by the following formula29 RPS = 1- (Percent mortality in treated group/ Percent mortality in control group) × 100
Statistical analysis
ANOVA followed by Duncan’s multiple range test30 was applied to find out significant differences among dietary treatments Statistical significance was tested at a probability value of P<0.05. The statistical tests were performed using SPSS Version 11.5 for Windows.
Effect of levamisole on growth and digestibility
At the end of experiment, all groups receiving levamisole supplemented diets revealed significant increase in the body weight gain, specific growth rate (SGR), protein efficiency ratio (PER), gross conversion efficiency (GCE) and apparent protein digestibility (APD). A significant decrease in feed conversion ratio (FCR) in comparison with control group was found. These results are demonstrated in table (3) and figure (1). After 60 days, mean digestive enzyme activities of all levamisole treated groups were significantly different (P<0.05) with that of the control. The protease activity was remarkably higher (P<0.05) in L2 (2.33±0.05) compared with control and other treatments. Amylase and cellulase, assays showed significantly higher (P<0.05) activity in treatment L2 fed fishes as compared to the rest.
|
Growth parameters |
Dietary treatments |
|||
|
LC (control) |
L1 (125 mg) |
L2 (250 mg) |
L3 (500 mg) |
|
|
Initial weight (g) |
1.67±0.01A |
1.68±0.016A |
1.69±0.02A |
1.69±0.02A |
|
Final weight (g) |
4.39±0.06C |
4.83±0.08B |
5.44±0.08A |
5.00±0.09B |
|
Live weight gain (g) |
2.72±0.05C |
3.15±0.10B |
3.75±0.06A |
3.31±0.07B |
|
Pre-challenge Survival rate (%) |
96.00±2.60A |
100A |
100A |
100A |
|
Growth (%) gain in BW |
162.50±3.18C |
187.70±8.09B |
222.00±2.19A |
196.15±3.29B |
|
Growth day¯¹ (%) in BW |
1.50±0.016C |
1.61±0.03B |
1.75±0.08A |
1.65±0.01B |
|
Specific growth rate (SGR) (% BW d-1) |
0.69±0.008C |
0.76±0.02B |
0.84±0.004A |
0.78±0.008B |
|
Feed conversion ratio (FCR) |
3.62 ± 0.05A |
3.53 ± 0.10AB |
3.10 ± 0.08C |
3.31 ± 0.03BC |
|
Gross conversion efficiency (GCE) |
0.27 ± 0.003C |
0.28 ± 0.008BC |
0.32 ±0.009A |
0.30± 0.003AB |
|
Protein efficiency ratio (PER) |
0.69± 0.009C |
0.70± 0.02B |
0.80 ± 0.02A |
0.75 ± 0.007AB |
|
Apparent Protein Digestibility (%) |
71.2±0.72C |
74.26±0.63B |
79.80±0.72A |
76.10±0.51B |
|
Specific protease activity1 |
1.46±0.05C |
1.68±0.02B |
2.33±0.05A |
1.78±0.05B |
|
Specific amylase activity2 |
1.21±0.02C |
1.29±0.03C |
1.56±0.03A |
1.43±0.02B |
|
Specific cellulase activity3 |
0.86±0.02D |
1.06±0.08C |
1.18±0.04A |
0.90±0.04B |
Table 3 Growth performances and intestinal enzyme activities of Cirrhinus mrigala fed on diets containing varying proportions of levamisole
All values are Mean ± S.E of mean.
1mg of tyrosine liberated mg of protein¯¹ h¯¹
mg of maltose liberated mg of protein¯¹ h¯¹
3mg of glucose liberated mg of protein¯¹ h¯¹
Means with different letters in the same row are significantly (P<0.05) different.
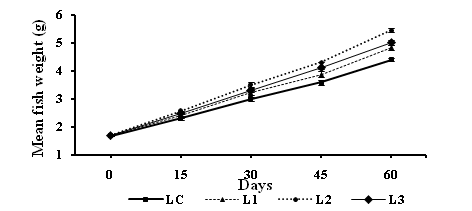
Figure 1 Increase in mean fish weight (g) (Mean ±S.E of mean) of Cirrhinus mrigala fed on diets supplemented with varying proportions of levamisole (LC=control, L1=125 mg, L2=250 mg and L3=500 mg of diet) from day 15 to 60.
Effect of experimental diets on water quality characteristics
The data on water quality characteristics pertaining to four dietary treatments is presented in table-4. In general, significantly (P<0.05) low values in total ammonia excretion and reactive phosphate production (mg Kg¯¹ BW d¯¹) were recorded in fish fed on diet L2. However, peak values of ammonia excretion occurred approximately 2 h after feed was given to fish and second peak at 8 h after feeding while o-PO4 production showed an initial high level at 4 h post feeding and second peak at 10 h post feeding (Fig-2A and 2B).
|
Physiochemical Parameters |
Dietary Treatments |
|||
|
LC (control) |
L1 (125 mg) |
L2 (250 mg) |
L3 (500 mg) |
|
|
Dissolved oxygen (mgL-1) |
7.10±0.07A |
6.85±0.07B |
6.77±0.05B |
7.22±0.05A |
|
pH |
7.71±0.03A |
7.72±0.03A |
7.64±0.05A |
7.62±0.04A |
|
Conductivity (µ mho cm-1) |
660.00±5.78B |
654.00±3.51A |
705.00±8.89A |
713.00±5.18B |
|
Temperature (°C) |
25.70±0.33A |
26.90±0.54A |
28.00±0.35A |
27.80±0.32A |
|
Alkalinity |
145.00±2.08B |
168.00±1.15A |
151.00±1.20B |
160.60±1.30A |
|
Chloride( mg L-1 ) |
30.60±0.83A |
28.50±2.13A |
25.90±2.05A |
27.16±2.17A |
|
Calcium (mgL-1) |
22.32±0.74C |
26.43±0.34B |
28.70±0.58A |
24.61±0.57BC |
|
Total dissolved solids |
601.00±11.54A |
584.00±9.08B |
546.00±8.15C |
571.00±10.40BC |
|
Total ammonia excretion (mg Kg-1 BW day-1) |
1572.30±16.70A |
1266.00±13.59B |
906.00±10.09D |
1064.00±13.56C |
|
Total phosphate production (mg Kg-1 BW day-1) |
645.00±9.93A |
608.30±10.10B |
526.00±13.13D |
574.00±9.27C |
Table 4 Effect of fish fed with diet containing different proportion of levamsiole supplementation on water quality characteristics (Mean values of data analysed at fortnightly intervals)
All values are Mean ± S.E of mean.
Means with different letters in the same row are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
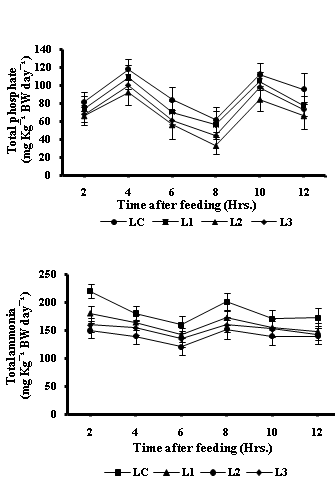
Figure 2 Post prandial excretory patterns of orthophosphate (2A) and total ammonia (2B), (mg kg-1 body weight per day of fish) in holding water for fish Cirrhinus mrigala in different dietary treatments containing varying proportion of levamisole (LC=control, L1=125 mg, L2=250 mg and L3=500 mg of diet).
Carcass composition
After 60 days feeding trial initial and final carcass composition of Cirrhinus mrigala in relation to various feeds is presented in (Table 5). The carcass composition of the test animals revealed a significant (P<0.05) increase in the final carcass protein and lipid over the initial carcass protein and lipid. Significantly highest carcass protein (16.4±0.20) and lipid (7.46±0.2) was recorded in treatment L2 fed fishes as compare to other experimental and control feeds. However, no significant (P<0.05) variations were observed in crude ash (%) of carcass of fishes fed on different diets.
|
Proximate composition |
Initial value |
Dietary treatments |
|||
|
LC (control) |
L1 (125 mg) |
L2 (250 mg) |
L3 (500 mg) |
||
|
Moisture (%) |
70.00±0.53 |
68.40±0.45A |
67.10±0.28B |
64.70±0.35C |
65.60±0.23C |
|
Crude protein (%) |
10.40±0.38 |
11.62±0.18D |
14.91±0.46B |
16.40±0.20A |
13.20±0.35C |
|
Crude fat (%) |
5.31±0.18 |
5.80±0.15C |
6.71±0.14B |
7.46±0.20A |
7.38±0.07A |
|
Crude ash (%) |
2.25±0.14 |
3.50±0.11A |
3.01±0.30AB |
2.83±0.16C |
2.93±0.22BC |
|
Nitrogen free extract (%) |
12.03±0.22 |
10.68±0.41A |
8.25±0.52B |
8.16±0.48B |
10.88±0.29A |
|
Gross energy (kJ/g) |
6.36±0.12 |
7.05±0.03C |
7.90±0.05B |
8.37±0.05A |
7.80±0.11B |
|
Phosphorous (%) |
0.54±0.01 |
0.51±0.05C |
0.59±0.03C |
0.65±0.012B |
0.73±0.02A |
Table 5 Proximate carcass composition of Cirrhinus mrigala fed on diets containing varying proportions of levamisole
All values are Mean±S.E of mean.
Means with different letters in the same row are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
Haematological Parameters (Total Erythrocyte and Leukocyte Count)
Levamsiole incorporation in C. mrigala diet in present studies resulted in elevated level in total erythrocyte and total leucocyte count. The Total erythrocyte count was significantly higher (P<0.05) in fishes fed on diet L2 (1.93±0.04) than in the control treatment LC (1.22±0.01). Also, significant increase (P<0.05) in WBC count was observed in fishes of treatment L2 (51.26 ±0.63) than in control treatment LC (22.06±0.73) (Table 6). However, erythrocyte count declined while TLC increased in the levamisole treated group in the after a challenge trial as compared to control.
|
Treatments |
Haematological Parameters |
|||
|
TEC (106mm3) |
TLC (103mm3) |
|||
|
Pre Challenge |
Post Challenge |
Pre challenge |
Post Challenge |
|
|
LC (control) |
1.22±0.0D |
1.08±0.015D |
22.06±0.73D |
23.46±0.35D |
|
L1 (150 mg) |
1.48±0.0C |
1.31±0.016C |
31.76±0.62C |
37.03±0.64C |
|
L2 (250 mg) |
1.93±0.0A |
1.80±0.04A |
51.26±0.63A |
54.32±0.64A |
|
L3 (500 mg) |
1.66±0.0B |
1.55±0.02B |
40.76±0.96B |
42.66±0.77B |
Table 6 Haematological parameters of Cirrhinus mrigala fed on diet containing various proportions of levamisole
TEC: Total erythrocyte count
TLC: Total leucocyte count
Means with different letters in the same column are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
Phagocytic responses
Phagocytic activity (PA) and phagocytic index (PI) of fish fed garlic diets were significantly higher than those of fish fed the control diet for 60 days (Table 7). The relative PA and PI levels (compared to the control group) of fish fed diets L1, L2 and L3 for 60 days increased by 64.3±3.75% and 1.68±0.07%; 80.31±0.96% and 2.35±0.03%; 72.40±1.62% and 1.76±0.05%, respectively.
|
Peripheral Blood Monocytes |
Treatments |
||||
|
Phagocytic index |
Phagocytic ratio (%) |
Bacteria within phagocytes |
No. of ingested phagocytes |
Total no. of phagocytes |
|
|
1.54±0.06C |
60.63±1.27C |
107.00±7.37C |
42.00±1.53C |
69.30±2.96C |
LC (control) |
|
1.68±0.07C |
64.35±3.75C |
127.00±9.6C |
48.30±2.18C |
75.30±3.18B |
L1 (150 mg) |
|
2.35±0.03A |
80.31±0.96A |
218.60±4.06A |
74.60±1.76A |
93.00±2.52A |
L2 (250 mg) |
|
1.76±0.05B |
72.40±1.62B |
160.30±8.38B |
66.00±2.89B |
91.00±2.08A |
L3 (500 mg) |
Table 7 Effect of levamisole and their interaction on Phagocytic ratio and Phagocytic index (%) of Cirrhinus mrigala
Means with different letters in the same column are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
NBT assay or Respiratory burst activity
The respiratory burst activities of cell in treated increased significantly (P<0.05) compared with the control fish (Table 8). Maximum increase in the NBT reduction value was observed in treatment L2 containing levamisole @ 250 mg kg-1 of diet.
|
S. No. |
Treatments |
The respiratory burst activity (O.D. at 540 nm) |
|
1 |
LC (control) |
0.39±0.01D |
|
2 |
L1 (150 mg) |
0.48±0.01C |
|
3 |
L2 (250 mg) |
0.71±0.01A |
|
4 |
L3 (500 mg) |
0.55±0.02B |
Table 8 The respiratory burst activity of Cirrhinus mrigala fed on diet containing various proportions of levamisole
Means with different letters in the same column are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
Relative percentage survival after challenge test
The challenge test for 10 days was performed after the feeding trial revealing that long term oral administration of levamisole supplemented feed enhanced the resistance of Cirrhinus mrigala to bacterial infection (Table 9). Water quality parameters were analysed on day one and day 5 of challenge trial. No significant variations were observed hence data not shown. After challenge with Aeromonas hydrophila, the first mortality was recorded after 48h. Significantly higher (P<0.05) post-challenge survival rates were observed in the fish groups fed diets containing levamisole @ 250 mg kg-1 of feed (86.35%) followed by L3 (50.06%) and L1 (31.78%) while the fish fed with control diet exhibited the highest mortality (73.3±6.67%). However, pre-challenge survival rate (%) during the feeding trial was high in all dietary treatments and slight mortality occurred only during the initial days of experiment.
|
S. No. |
Treatments |
Percentage Mortality (%) |
Relative Percent Survival (%) |
|
1 |
LC (control) |
73.30±6.67A |
|
|
2 |
L1 (150 mg) |
50.00±5.78B |
31.78 |
|
3 |
L2 (250 mg) |
10.00±5.78C |
86.35 |
|
4 |
L3 (500 mg) |
36.60±3.33B |
50.06 |
Table 9 Percentage mortality and Relative Per cent Survival (RPS) of Cirrhinus mrigala in a challenge trial with Aeromonas hydrophila for 10 days
Means with different letters in the same column are significantly (P<0.05) different.
(Data were analyzed by Duncan’s Multiple Range test)
The growth trial conducted in present study showed significantly (P<0.05) enhanced growth and feed efficiency (in terms of SGR, PER, GCR, APD, etc) of C. mrigala after 60 days of consuming the diet with different concentration of levamisole. Maqsood et al.10 also reported that upon dietary levamisole supplementation (250 mg levamisole Kg-1 of diet) the growth parameters of Cyprinus carpio were significantly enhanced when compared with the fish fed on control diet. Similar findings were reported by Misra et al. & Mulero et al.31-33 for gilthead seabream reported that the dietary intake of levamisole had a significant impact on the size and weight of fish throughout the experiment when compared with control. In a study by Li et al.34 on juvenile hybrid striped bass, low doses of levamisole (<500 mg kg-1 of diet) significantly enhanced feed efficiency and growth by approximately 10% after 3 weeks of feeding as compared to fish fed on basal diets. In the present study 37.86 % increase in growth has been observed by feeding the fish on levamisole (@ 250 mg kg-1) supplemented diets in comparison to controls. From these results it could be said that levamisole has potent growth promoting effect and may act as alternative approach to enhance fish health.
In aquaculture, water quality deteriorates mainly due to accumulation of metabolic wastes such as ammonia and orthophosphate excretion in the holding water. In the present studies the excretion of metabolites i.e., N-NH4 and o-PO4 in the holding water also increased with the increase in the inclusion level of levimasole dose. This may again be attributed to low feed utilization and when dietary utilization is low, deamination of unutilized feed protein occurs and excretion of metabolites in the holding water increases above the optimum. Thus when the value of the immunostimulant exceeds the optimum limit, there is negative impact on nutritive physiology decreasing growth performance and increasing excretion of metabolites in holding water.
Our result clearly indicates the enhancement in certain carcass composition in all immunostimulants treated fishes. Perhaps high nutrient value in carcass was due to the increased enzymatic activity in the gut and there by nutrients was utilized properly and spends for the growth making overall well-being for the fishes.
Levamsiole incorporation in C. mrigala diet in present studies resulted in elevated level in total erythrocyte and total leucocyte count. However, erythrocyte count declined after challenge with pathogen may be due to effect of pathogen but this effect was least in the fishes where levimasole was used at optimum dose corresponding significantly with other immune response parameters indicating that supplementation of levimasole at optimum levels can significantly reduce the effect of pathogen. These findings are in agreement with that of Bruno and Munro35 who reported that the experimental infection of rainbow trout and Atlantic salmon with Renibacterium salmoninarum and the subsequent progression of Bacterial Kidney Disease (BKD) resulted in the significant decline in TEC levels. Similar findings were also reported by Mulero et al. Kumari & Sahoo.33,9-11 Likewise, there was an increase in TLC in the levamisole treated group in the after a challenge trial as compared to control which could be attributed to the activation of immune response against the bacterial invasion which was significantly (P<0.05) high in fish group fed on diet containing levamisole in the proportion of 250 mg kg-1 of diet. Similar findings of significantly enhanced total leucocyte level in carp fed with levamisole @250 mg kg-1 when compared to control were reported by Maqsood et al. & Misra et al.11 also observed significantly (P<0.05) high WBC count in fish administered with low and medium dosages of levamisole when compared to control group.
Many studies have reported capability of levamisole in enhancing immune responses and/or reducing losses from bacteria and/or parasitic infections in different species such as carp,8,10 gilthead sea bream,33 rainbow trout,14,36 Atlantic salmon,37,38 rohu,39,40,11 hybrid striped bass,34 catfish.12
Levamisole induced significant (P<0.05) increase in blood phagocytic activities in all treatment groups as compared to control in the present studies. Wijendra & Pathiratne40 also observed significantly elevated levels of total phagocytic activity, phagocytic index in levamisole treated Labeo rohita compared to that of the respective control fish post 21 days of treatment. Similar results were obtained by Ispir & Yonar36 in Rainbow Trout (Oncorhynchus mykiss) where fish exposed to different concentrations of levamisole showed significantly higher activity of phagocytic cells than that in controls. Kajita et al.41 in a study observed that activation of alternative complement pathway by levamisole may attribute to enhanced phagocytosis, chemiluminescence responses and natural killer cell activity in rainbow trout, Oncorhynchus mykiss (Walbaum). Misra et al.11 also found significant (P<0.05) increase in phagocytic ratio in different treatment groups of Labeo rohita up to 42nd day of immunomodualtion trial. On all the assay days, it was marked that medium dose of levamisole could result in higher phagocytic ratio compared to other group of fish. In our study too, maximum phagocytic activity was reported in L2 fed group containing levamisole @ 250 mg kg-1 clearly indicating a dose dependent response of immunostimulant levimasole.
Phagocytes produce huge quantities of superoxide anion during phagocytosis or upon stimulation which can be reduced by NBT. The NBT reduction product obtained after reaction with superoxides is hence a good indicator of the health status or the immunization effectiveness in fish Anderson et al.42 which was also observed in present study. Similar results were obtained by Gopalakannan & Arul32 for Cyprinus carpio. Kumari & Sahoo9 also reported significantly high respiratory burst (NBT) activity in levamisole fed group as compared to the CYP-treated control group. Rairakhwada et al.43 also showed that the NBT values for levamisole fed fish were higher when compared with the control fish. A higher NBT activity was detected in Puntius gonionotus (Japanese carp) following injection with A. hydrophila.44 Sharp & Secombes45 suggested that increased respiratory burst activity can be correlated with increased bacterial pathogen killing activity of phagocytes.
A high survival rate was reported on levamisole treated fish group after a 10 day challenge with pathogenic A. hydrophila. Similar findings were reported by Gopalakannan & Arul32 after challenge trial of immunostimulant supplemented feed fed Cyprinus carpio with Aeromonas hydrophila. Baba et al.8 also reported that survival rate after challenging the fish with Aeromonas hydrophila was higher in common carp treated with levamisole. Levamisole has also been found to be a possible modulator of the immune response by eliciting resistance after challenge trial in Cyprinus carpio46,8 Oncohrynchus mykiss,42 Sparus aurata,33 Clarias batrachus,9 Morone chrysops×Morone saxatilis.34
Results of the present investigation reveal that a dose of 250 mg levamisole kg¯¹ diet is optimum to stimulate the immune function of Cirrhinus mrigala same dose oflevamisole was equally effective in stimulating thegrowth. However efficacy of levamisole and its study on cellular and molecular mechanisms is subject for further research.
None.
None.

©2016 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.