Journal of
eISSN: 2378-3184


Research Article Volume 13 Issue 2
1Lagoon Ecology and Aquaculture Laboratory (LEALab), Orbetello Pesca Lagunare Company, Italy
2University of Siena, Dep. Economics and Statistics, Italy
Correspondence: Mauro Lenzi, Lagoon Ecology and Aquaculture Laboratory (LEALab), Orbetello Pesca Lagunare Company v. G. Leopardi 9, 58015 Orbetello, Italy
Received: July 02, 2024 | Published: July 18, 2024
Citation: Lenzi M, Leporatti Persiano M, D’Agostino A. Growth tests of Gongolaria barbata (Ochrophyta Sargassaceae), a native species producing pleustophitic blooms in a hypertrophic Mediterranean lagoon. J Aquac Mar Biol. 2024;13(2):71-78. DOI: 10.15406/jamb.2024.13.00399
In the Orbetello lagoon (Italy), the Ochrophyta Gongolaria barbata has shown in recent years a tendency to form vegetative blooms in the winter-spring period, with decay in midsummer. Growth tests were conducted in the laboratory and the field to understand the reasons for these unexpectedly opportunistic characteristics of this species. In the laboratory, different salinities and concentrations of P and N and N/P molar ratios were tested using nitrate and ammonium nitrogen. In the field, tests were carried out in three lagoon areas with different macroalgal development scenarios dominated by the Chlorophyta Chaetomorpha linum, analysing the water and using the physico-chemical data provided continuously by two stations equipped with multi-parameter probes.
Laboratory tests showed: Ochrophyta's ability to grow in oligo-, meso- and hypertrophic conditions, both for N and P, even for very unbalanced N/P molar ratios, although intolerance to ammonium nitrogen-dominant conditions was observed; wide tolerance to salinity; increased growth for temperatures between 26°C and 30°C. In the lagoon, tests showed increased growth for strongly nitrate-nitrogen-dominated hypertrophic conditions, in areas with dense outcropping mats of C. linum, where G. barbata overgrew. SGRs were not elevated either in the laboratory or the lagoon, compared to those of C. linum. SRG values were significantly higher in the West basin than in the East basin.
The hypothesis we draw from the results is that the species, previously confined with modest biomass development in oligo-mesotrophic areas close to the sea mouth, was able to develop in bloom when the lagoon reached hypertrophic conditions, i.e. when nutrient availability became unlimited. In the past, it probably could not compete for the resources with opportunistic and nitrophilous species with high nutrient uptake capacities. G. barbata develops, in fact, above all in the over-layer of dense Chlorophyta mats, which by creating anoxic conditions in the substratum allow substantial nutrient releases that not only support the mat itself but also the development of the Ochrophyta. In winter-spring, ammonium releases are oxidised to nitrate in the pathway from the bottom to the surface of the water column, but the nitrification process fails in summer and its mats decay as a result of the increased summer decay of Chlorophyta mats, which results in the dominance of ammonia nitrogen and releases of hydrogen sulphide.
Keywords: Gongolaria barbata, eutrophication, lagoon, macroalgal bloom, opportunistic species
Coastal lagoons are highly stressed transitional areas due to the wide variability of environmental conditions that make them prohibitive for many species of both continental and marine origin, so few species can live and grow, but those few colonise with large numbers of individuals. Lagoons are food-rich, mesotrophic environments with a natural tendency to eutrophication. This is even more true for non-tidal lagoons, where the tidal excursion is modest and has little influence on the water exchange of the basin.
The condition of eutrophication and, more recently, that of hypertrophy, due to the intensification of human activities, determine an imbalance in trophic dynamics, altering, even more than is already the case due to the nature of the lagoon basin itself, food networks and relations in the flora-fauna communities.
The euryhaline and eurythermic lagoon biocenosis (LEE), under eutrophic conditions, expresses facies dominated by opportunistic macroalgae and, in even more extreme cases, intense microphytic developments.1 Lagoon communities are structurally simpler and have fewer species than coastal marine ecosystems and those in continental waters, but have a high resilience that allows them to return to equilibrium after stress that has produced changes.2 However, in the last decade, in addition to the eutrophic/hypertrophic conditions to which many lagoon basins are subjected as a result of the increasing anthropization of the territory, climate change has been added, with increasingly frequent heat-waves.3 Lagoons, which are mostly laminar environments, especially non-tidal lagoons, although highly resilient, are more susceptible to temperature rises than deep basins with high turnover. A condition, this, of further selection for submerged communities.
Eutrophication, ongoing climate change, and numerous other human-induced alterations to ecosystems have led to the uncontrolled development of numerous species, some already known to be cosmopolitan and opportunistic, others non-native, which probably only in recent times, given the weakness and rarefaction of ecosystem trophic networks, have found a way to establish themselves and, in some cases, produce blooms, and still others native species, unexpectedly opportunistic.
This study dealt with a native species, the Ochrophyta Gongolaria barbata, which in recent years displayed an increasingly important vegetative development and spread in the Orbetello Lagoon (Tuscany, Italy). This Tyrrhenian lagoon is prone to macroalgal development, essentially sustained by cosmopolitan and nitrophilous species, but G. barbata is not the first native species that is neither notoriously opportunistic nor nitrophilous to produce blooms, it has already happened in the past with two typically marine Rhodophyta that can also develop in lagoons, Alsidium corallinum e Sphaerococcus coronopifolius. These episodes are indicative of how this lagoon environment is extremely variable and how some species can manifest an unexpectedly opportunistic character.
The aim of this study was to try to clarify why G. barbata has recently developed so significantly, and therefore what are the optimal growth conditions for this species, using growth tests in the laboratory and in a lagoon environment. Knowledge of these basic elements is indispensable to establish possible techniques to control blooms and understand the eutrophic dynamics of the lagoon basin.
Study area
The Orbetello lagoon is a shallow, non-tidal, hypertrophic environment with low water-turnover. It is located along the Tyrrhenian coast (42◦ 25’ –42◦ 29′ N; 11◦ 10′ –11◦ 17′ E) and consists of two communicating basins, West and East, of 15.25 km2 and 10.00 km2, respectively. This lagoon has three canals of communication with the sea, two in the West basin (SLC1; SLC2) and one in the East basin (SLC3) (Figure 1). Because water exchange with the sea is relatively difficult, salinity varies between the wettest seasons, fall and spring, and the dry summer season, between 25 and 40 (practical salinity scale). Hypertrophic condition is due to the wastewater of two land-based fish-farms, discharged in the easternmost sector of the lagoon (Figure 1), intermittent agricultural run-off flow and nutritional sediment stores coming from the urban and productive activities wastewaters discharged in the past.4,5 Orbetello lagoon is subject to intense macroalgal blooms, with wide fluctuations in the dominance of species: in the 1980s, dominated Gracilariopsis longissima (previously named Gracilaria verrucosa), in both West and East basins, and Ulva rigida, in East;6 in the early 1990s, Cladophora vagabunda, with an impressive development in 1992;7 later, more steadily, dominance shifted to Chaetomorpha linum, in both basins,8 but with substantial developments localised in the West, of Alsidium corallinum in 20079 and from 2013 of Sphaerococcus coronopifolius, only in East basin.10
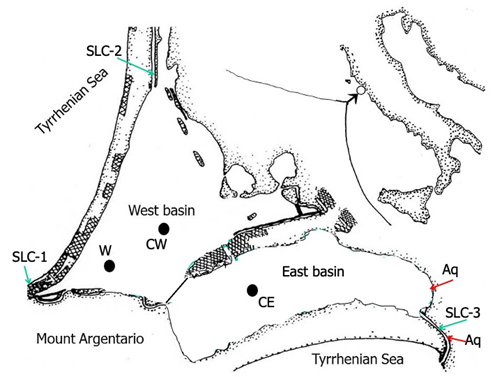
Figure 1 The Orbetello Lagoon, placed along the Tuscany Tyrrhenian coast, divided into two communicating basins, West and East. SLC 1, 2, 3, sea-lagoon canals and sea-water pumping stations (SLC 1, 2); W, CW, CE, west, central west and central east stations for the macro algal growth test; CW, CE, control units equipped with multi parametric probes; Aq, aquaculture waste waters.
The environmental management of this lagoon is based on three mitigation operations: macroalgal harvesting; oxidation of sediments with a high organic load by their resuspension in the water column; forced water turnover by seawater pumping for 5-6 months per year. This latter environmental mitigation system, although not very effective in terms of improving eutrophication, is activated by two pumping stations located in SLC1 and SLC2, which cause water masses to move from West to East (input of 12 m3 s-1; theoretical daily exchange of 4% of the total lagoon volume). The pumping excludes the natural circulation with the sea, the injected water is pushed towards the only mouth remaining open to free circulation, SLC3, at the east end of the East basin (Figure 1).
Gongolaria barbata
The Ochrophyta Sargassaceae Gongolaria barbata (Stackhouse) Kuntze, (synonyms: Treptacantha barbata (Stackhouse) Orellana & Sansón, 2019; Cystoseira barbata (Stackhouse) C. Agardh, 1820), is a widespread species, found in the Mediterranean, Black Sea, Portugal, Morocco, India, Pakistan.11 A species with broad polymorphism, it has numerous infraspecific taxa and has recently been redefined as Gongolaria barbata f. aurantia (Kützing) Falace, Alongi et Kaleb comb. nov.12 Here it is simply referred to as Gongolaria barbata.
This Ochrophyta is a eurytherm species that can tolerate temperatures from very low to >30°C,13 is found anchored to rocks, near harbours, on artefacts and populates coastal lagoons where it is predominantly pleustophytic, freely floating on the bottom. It is considered a good indicator of heavy metal pollution14 and organic contaminants.15 Along the Crimean coast, G. barbata can grow abundantly, absorb petroleum hydrocarbons and be a source of secondary pollution once masses accumulate under the coast,16 In addition, a fat content of 2% of dry weight was estimated in the same area, with a tendency to increase in algae grown in areas with a higher degree of pollution.17 However, it is being looked at as a possible mineral source, rich in macro- and micro-nutrients with a low content of toxic elements,18 and for the extraction of fucoxanthin, due to its antioxidant, anti-cancer and anti-diabetic properties.19
In the Orbetello lagoon, Gongolaria barbata is present in the floristic lists of the 1970s20 and the 1980s6 as Cystoseira barbata, but only localised near the sea-lagoon communication canal SLC1 (Figure 1), i.e. in the area most affected by seawater exchange. It is always found floating on the bottom in bento-pleustophytic conditions, and in spring the thalli may partially emerge to the surface in acro-bento-pleustophytic conditions. Towards the end of the 1990s, this species spread to the innermost areas of the lagoon,21 forming small pleustophytic masses arranged in patches among the Gracilariaceae, especially in summer, in the West basin. We observed this species began to populate with progressively more abundant pleustophytic wisps in both West and East basins from 2016 onwards, mixing with the dense mat of the Chlorophyta Chaetomorpha linum, which had meanwhile become dominant in this lagoon. Since 2022, G. barbata has consistently increased its biomass, constituting between 5% and 12% of the lagoon's entire macroalgal biomass in the most intense spring standing crop, which averages around 60,000 tonnes wet weight.
To understand the growth dynamics of this Ochrophyta, several laboratory growth tests were conducted. In 2023, between March and June, the G. barbata growth was tested under conditions of 1) salinity variability, 2) nitrogen concentration variability and 3) phosphorus concentration variability. In May 2024, a laboratory test of the growth of the species under conditions of varying nutrient concentration and salinity was repeated, and a growth field test was conducted in three different areas of the Orbetello lagoon.
For the laboratory tests, conducted at room temperature and with solar light radiation, the chemical-physical variables were measured by OxyGuard Handy Mk III oximeter for dissolved oxygen, DELTA OHM HD8705 for temperature and pH, ATAGO S/Mill refractometer for salinity (practical salinity scale), and HI 97500 Luxmeter for illuminance.
Test 2023. Salinity
G. barbata thalli were collected in the lagoon and housed in the laboratory for 5 days. Subsequently, 3 thalli fragments, dried with adsorbing paper and weighed (1.99 g, 2.46 g and 2.38 g) were placed in as many 1L cylinders (weights, s40, s27, s19 respectively), into which artificial seawater of different salinities had been added: s40 at salinity 40; s27 at salinity 27; s19 at salinity 19. The test was conducted from March 17 to April 6 and the weighing of the thalli was carried out every 5 days. Nutrients were added using a solution of Walne medium,22 in the same quantities (Table 1) in all 4 cylinders, at the beginning of the test, after 5 days and after 10 days.
Test 2023. Nitrogen and phosphorous increases
9 thalli fragments (ranging from 1.74 g to 2.96 g), dried with adsorbing paper and weighed, were placed in as many 1L cylinders (microcosms A, A2, B, B2, C, C2, D, E, F) with artificial seawater at salinity 37 and in which varying amounts of nutrients were added (Table 1). The growth test was conducted from April 17 to May 1, for cylinders A, B, C, from March 22 to April 6, for cylinders A2, B2, C2, and from April 18 to May 6, for cylinders D, E, F, weighing every 5 days.
|
N-NH4 |
N-NO2 |
N-NO3 |
DIN |
SRP |
DIN/SRP |
SGR |
||
|
%gww d-1 |
||||||||
|
2023 lab. |
s19 |
0.60 |
0.06 |
10.00 |
0.767 |
|||
|
s40 |
0.60 |
0.06 |
10.00 |
0.199 |
||||
|
s27 |
0.60 |
0.06 |
10.00 |
0.813 |
||||
|
A |
0.12 |
0.02 |
6.00 |
1.139 |
||||
|
B |
18.70 |
0.06 |
312.00 |
1.228 |
||||
|
C |
11.80 |
0.06 |
197.00 |
-0.518 |
||||
|
A2 |
0.60 |
0.06 |
10.00 |
1.168 |
||||
|
B2 |
748.00 |
0.06 |
12467.00 |
1.097 |
||||
|
C2 |
706.00 |
0.06 |
11767.00 |
-3.478 |
||||
|
D |
0.60 |
1.43 |
0.42 |
1.100 |
||||
|
E |
0.60 |
2.86 |
0.21 |
1.047 |
||||
|
F |
0.60 |
5.71 |
0.11 |
0.916 |
||||
|
2024 lab. |
G |
0.60 |
0.06 |
10.00 |
1.181 |
|||
|
H |
0.60 |
0.06 |
10.00 |
1.192 |
||||
|
I |
1.20 |
0.12 |
10.00 |
0.903 |
||||
|
L |
2.40 |
0.24 |
10.00 |
1.066 |
||||
|
2024 field |
W |
27.64 |
0.00 |
21.00 |
48.64 |
0.97 |
50.25 |
|
|
W1 |
|
|
|
|
|
|
0.320 |
|
|
W2 |
0.682 |
|||||||
|
W3 |
1.260 |
|||||||
|
W4 |
0.920 |
|||||||
|
W5 |
1.000 |
|||||||
|
W6 |
0.904 |
|||||||
|
|
CW |
26.64 |
0.00 |
211.29 |
237.93 |
3.23 |
73.75 |
|
|
CW1 |
|
|
|
|
|
|
1.594 |
|
|
CW2 |
1.442 |
|||||||
|
CW3 |
0.820 |
|||||||
|
CW4 |
2.200 |
|||||||
|
CW5 |
0.620 |
|||||||
|
CW6 |
1.500 |
|||||||
|
|
CE |
8.14 |
0.14 |
17.42 |
25.70 |
0.65 |
39.84 |
|
|
CE1 |
|
|
|
|
|
|
0.166 |
|
|
CE2 |
0.214 |
|||||||
|
CE3 |
0.104 |
|||||||
|
CE4 |
0.660 |
|||||||
|
CE5 |
0.240 |
|||||||
|
CE6 |
0.144 |
Table 1 Results of the specific growth rate (SGR) of G. barbata in the laboratory tests of artificial nutrient enrichment and salinity changes, and in the lagoon test at stations
W, CW, CE. N-NH4 ammonium nitrogen; N-NO2, nitrite nitrogen; N-NO3 nitrate nitrogen; DIN, dissolved inorganic nitrogen; SRP, soluble reactive phosphorus; DIN/SRP, atomic ratio. W, CW, CE in bold, analytical determination of lagoon waters; SGR values >1, in bold.
Additions of N-NH4, N-NO3 and P-PO4 were carried out with solutions of NaNO3, NH4Cl and NaH2PO4•H2O.
Test 2024. Salinity and nutrient increase
In May 2024, 4 thalli fragments of G. barbata (3.14 g, 1.87 g, 2.73 g, 1.80 g), from thalli collected in the lagoon and housed for a few days in the laboratory, were placed in cylinders of 1L each (G, H, I, L, respectively) filled with artificial sea-water. The salinity was 16 for G and 37 for the other three. The same solution of the Walne medium was added in G and H, doubling it in I, and quadrupling it in L, always maintaining the same N/P molar ratio (Table 1). The same amounts of medium were added to the four microcosms on the seventh day of culture. The test was conducted from May 27 to June 19 and the weighings were carried out on the second, fifth, ninth, twelfth and twenty-third day of culture.
Lagoon test
In May 2024, G. barbata thalli were collected in the central area of the West Lagoon and acclimatised for 4 days in the laboratory at salinity and temperature conditions similar to those in the lagoon. Light intensity was artificially maintained at around 20 klux per 12 h d-1.
On the fifth day, 10 May 2024, 18 thalli fragments were randomly selected, dried with adsorbing paper and weighed (ranging from 5.61 g to 1.06 g). They were then attached to appropriately labelled plastic ropes and placed in a container with lagoon water for transport and transferred to the lagoon.
Three lagoon stations were chosen, 2 in the West basin (W, CW) and 1 in the East basin (CE) (Figure 1). At each station, 6 thalli fragments were placed, each attached to a bamboo rod by a plastic rope, and left floating in the middle of the water column. Each rope was labelled with the previously weighed fragment. The thallus of this species is sufficiently tenacious not to fragment easily, so it was not necessary to use special containers, which could have altered the flow of water inside and the light radiation.
The thalli fragments were left in the lagoon for five days, then recovered on 15 May 2024, each labelled, immersed in plastic baskets with lagoon water, and transferred to the laboratory, where they were dried with adsorbing paper and weighed.
During the same period as the growing thalli, duplicate water samples were collected at stations W, CW, and CE for nutrient determination. The water samples, refrigerated and transported to the laboratory, were filtered at 0.45, and then nitrate nitrogen (N-NO3), ammonia nitrogen (N-NH4), and soluble reactive phosphorus (SRP) were determined. The analyses were conducted according to APAT IRSA-CNR.23 Nitrate analysis was based on its reduction to nitrite and the subsequent formation of a coloured diazo-compound. Ammonium determination was based on a series of reactions, catalysed photochemically, that led to the formation of indophenol blue. SRP determination was based on the formation of a blue-coloured phosphomolybdic complex. The concentrations of the three coloured compounds were then assessed spectro photometrically.
The dissolved inorganic nitrogen (DIN = N-NH4+ + N-NO2- + N-NO3-) and the molar ratio DIN/RSP were also calculated.
CW and CE stations were placed near two control units equipped with multiparametric probes, for which data relating to the entire month of May of temperature (T; °C), pH, dissolved oxygen (DO; mg L-1; % saturation) salinity (s;), redox (Eh; mV) and direct radiation (in W m-2, converted to lux) were downloaded. The control units record data every 15 minutes.
Specific growth rate
The specific growth rate (SGR; gWW% d-1), expresses the daily growth rate of a population or biomass and here applied for G. barbata, is calculated by the equation:
Where lnW is the natural logarithm of the initial (Wi) and final (Wf) macroalgal weight, and t the time in days over which the growth was measured.
The days (t) were: 20 for s19, s40, s27; 15 for A, B, C, A2, B2, C2; 19 for D, E, F; 23 for G, H, I, L; 5 for lagoon stations W, CW and CE.
Statistical analysis
Kruskall-Wallis (KW) non-parametric test24 was used to detect differences among the three SGR groups from the G. barbata growth stations W, CW and CE. Dunn’s test (with Benjamini–Hochberg correction) was performed for pairwise multiple comparison.25 Statistical analysis was carried out with STATA software.26
Laboratory tests
In Figures 2, 3, 4 and 5, the growth of the G. barbata thalli is shown, respectively, due to variations in salinity, nitrate/ammonia nitrogen, and orthophosphate and for variation in salinity and increase in N and P balanced between them. SGRs relating to the various tests are shown in Table 1.
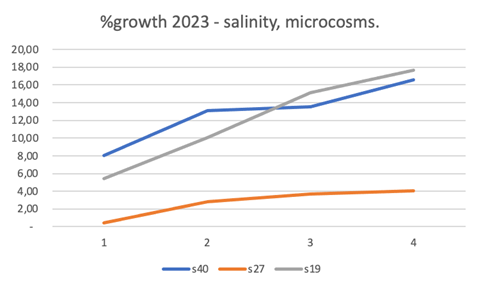
Figure 2 Results of the percentage of growth of Gongolaria barbata in the test conducted from March 17 to April 6, 2023, in microcosms with different salinity (19, 27, 40). Thalli weighing was carried out every 5 days (1-4, in abscissa). Temperature ranged between 19°C and 23°C, and daylight radiation between 6 and 30 klux.
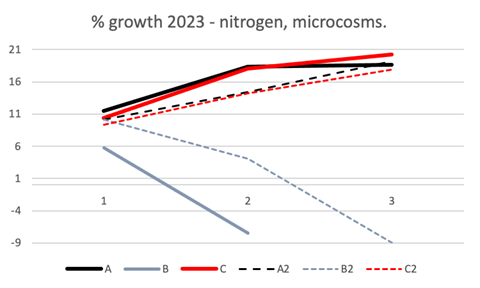
Figure 3 Results of the percentage of growth/decrease of Gongolaria barbata in the test conducted in 2023, varying nitrogen concentration, in microcosms at salinity 37, from April 17 to May 1, for cylinders A, B, C, from March 22 to April 6, for cylinders A2, B2, C2. Thalli weighing was carried out every 5 days (1-3, in abscissa). Temperature and daylight radiation ranged between 14°C and 19°C, and 6 klux and 38 klux for A2, B2, C2, and 17°C and 22°C and 9 klux and 52 klux for A, B, C, respectively.
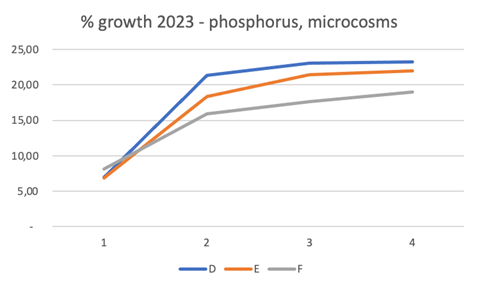
Figure 4 Results of the percentage of growth of Gongolaria barbata in the test conducted at varying phosphorus concentrations, in microcosms at salinity 37, from April 18 to May 6, 2023, for cylinders D, E, F, weighing every 5 days (1-4, in abscissa). Temperature and daylight radiation ranges were 20-22°C and 9-52 klux.
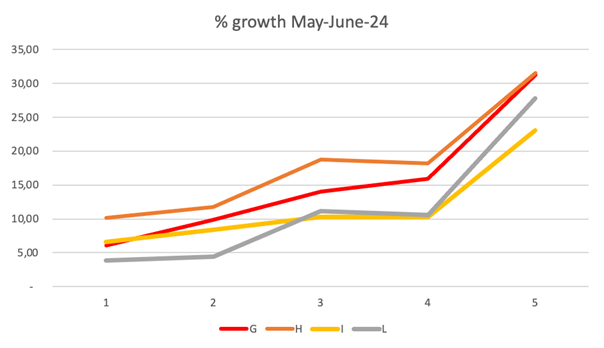
Figure 5 Results of the percentage of growth of Gongolaria barbata in the test conducted varying nutrient concentration with N/P ratio of 10, and with salinity 16 for microcosm G and 37 for microcosms H, I, L. The test was conducted from May 27 to June 19, 2024, and weighings were carried out on the second, fifth, ninth, twelfth and twenty-third days of culture (1-5, in abscissa). Temperature ranges was 19-24°C for the first 11 days, rising between 26°C and 30°C for the remaining days of the test (from 4 to 5 in abscissa). Daylight radiation was 9-57 klux.
From Figure 2, it can be observed that the species, in a modest nutritional condition, had good and constant growth especially at the lowest salinity (19) but also good at the highest salinity (40). The growth at intermediate salinity is constant but decidedly modest (27). The temperature fluctuated between 19°C and 23°C and the daylight radiation was between 6 and 30 klux.
Figure 5 shows good and constant growth, in a modest nutritional condition, both with low salinity (16) and high salinity. Lower results, although improving in the last days of the test when the temperature rose, were obtained in microcosms at salinity 37 with higher nutrient concentrations.
As regards the increases in nitrate and ammonium nitrogen, Figure 3 shows G. barbata did not seem to like nitrogen in a predominantly ammonium form, both at intermediate and high values (B, B2; 18 and 748 , respectively). The results were higher for predominantly nitrate-nitrogen, both at intermediate and high values (C, C2; 12 and 706, respectively), but also for low and balanced concentrations of nutrients with nitrate-nitrogen (A, A2). The daytime temperature and daylight radiation ranges were 14-19°C and 6-38 klux for A2, B2, C2 and 17-22°C and 9-52 klux for A, B, C, respectively.
P increases (Figure 4), despite unbalanced DIN/SRP molar ratios (microcosms D, E, F; 0.41, 0.21, 0.11, respectively), achieved relatively good growth, especially in the first 10 days of culture, subsequently the growth had flattened to a plateau, with better growth performance in the order D>E>F. The temperature and daylight radiation ranges were 20-22°C and 9-52 klux.
By using Walne's balanced medium in a constant 10 molar ratio with nitrate-nitrogen and orthophosphate, in microcosms G, H, I, and L, in increasing but relatively low doses (Figure 5), the best growth was achieved for the lowest concentration (G, H), followed by the intermediate (I), while the highest (L) started with greater difficulty, but then approached the values of the intermediate. The growth percentages are however relatively high (from 23% to 32% compared to the initial weight) at the end of the experience (23 days) for all 4 microcosms, regardless of salinity and nutrient concentration. Temperature varied between 19°C and 24°C for the first 11 days, rising between 26°C and 30°C for the remaining days of the test. Daylight radiation was 9-57 klux.
The SGRs for laboratory tests did not show high daily growth, as shown in Table 1. 1 %gww d-1 was slightly exceeded by the microcosms A, A2, B, B2, D, E, G, H, and L, which corresponded to low concentrations of nutrients or high, but from nitrate nitrogen and included the two low salinity microcosms.
Lagoon test
In Figure 6, the growth percentage of G. barbata thalli is reported for the fragments placed in the three stations of the Orbetello lagoon. Table 1 shows the results of the analytical determinations of the nutrients dissolved in the lagoon waters of the growth stations, and the SGR for each thalli fragment. Table 2 shows, for May 2024, the main chemical-physical variables transmitted by the control units close to the CW and CE algal growth stations. The daylight radiation in the 5 days examined varied from 89 klux to 545 klux.
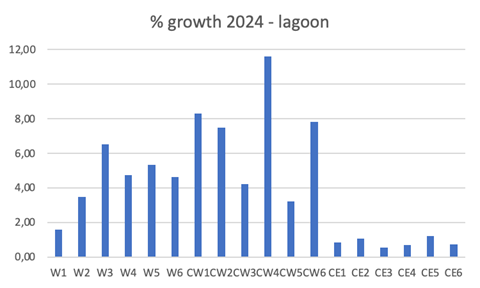
Figure 6 Results of the percentage of growth of Gongolaria barbata in the test conducted in three lagoon stations (W, CW, CE), where 6 thalli fragments per station (W 1-5, CW 1-5, CE 1-5) were placed for 5 days, from May 27 to June 19, 2024. Nutrients and physico-chemistry in Tables 1 and 2. Daylight radiation varied from 89 klux to 545 klux.
The CW station and the related control unit with the multiparametric probe were inside a vast and dense Chaetomorpha linum mat, outcropping in many places. G. barbata was arranged with frequent tufts on the surface of the mat. In station W, the macroalgal mats were mixed between C. linum and Valonia aegagropila, very dense for the former, while the latter occupied a compact but not very thick layer in large patches. G. barbata was distributed in a patchy pattern with abundant tufts overlying the previous mats. Even the vegetation at the CE station, adjacent to the related chemical-physical control unit, was made up of a mixture of C. linum and V. aegagropila both forming a layer on the bottom no greater than 10-15 cm. Here G. barbata was much less abundant than in CW and W.
Table 2 shows that there was good photosynthetic activity in the two lagoon basins, with high DO and pH as expected from high vegetative development. The environmental conditions were spring-like with temperatures and salinity still not high. However, in CW the redox potential was about half that of the other station.
Ammonia nitrogen concentration was relatively high in the West basin, more than 3 times that in the East basin, but nitrate nitrogen values were also high, with very high values in CW (Table 1). The same for soluble orthophosphates, which were elevated everywhere, but even more so in the West basin, especially in CW.
|
DO |
pH |
T |
Eh |
s |
s |
||
|
mg/L |
°C |
mV |
mS/cm |
pss |
|||
|
CW |
mean ± SD |
8.96 ± 1.95 |
8,81 ± 0.07 |
21.18 ± 1.97 |
123.69 ± 20.50 |
46.15 ± 1.27 |
|
|
median |
8.84 |
8.80 |
21.00 |
125.00 |
46.37 |
30.14 |
|
|
CE |
mean ± SD |
7.95 ± 1.32 |
8.69 ± 0.09 |
23.14 ± 2.20 |
223.28 ± 31.63 |
51.28 ± 1.03 |
|
|
median |
7.93 |
8.70 |
23.97 |
229.00 |
51.38 |
33.40 |
Table 2 Mean (± SD) and median for Dissolved oxygen (DO), pH, temperature (T), redox (Eh) and salinity (s) from multi parametric probes placed in the control units CW and CE, for May 2024
SGR exceeded 1 %gww d-1 in 2 out of 6 talli in W, in 4 out of 6 in CW and in none in CE. On average, SGR for W was 0.848 ± 0.291 %gww d-1, for CW 1.363 ± 0.521 %gww d-1, for CE 0.255 ± 0.187 %gww d-1. The mean percentage growth was 4.27 ± 1.70 % in W, 7.11 ± 3.03 % in CW, and 0.84 ± 0.25 % in CE. Kruskal-Wallis test results showed a significant difference between the SGR groups of W, CW and CE, at 5% (p = 0.0021). The Dunnett's test for multiple comparisons highlighted that the group that differed significantly from the other two was CE, while there was no significant difference between the W and CW groups (Table 3).
Although nutrients were well present in both basins and in all three selected algal growth stations, the DIN/SRP molar ratios were always decidedly high, in an imbalance in favour of nitrogen.
|
Dunnett's comparisons |
Test statistic value |
p |
|
W vs CW |
-0.9733 |
0.1652 |
|
CE vs W |
2.4333 |
0.0149 |
|
CE vs CW |
3.4067 |
0.0010 |
|
Kruskal-Wallis |
12.3160 |
0.0021 |
Table 3 Kruskal-Wallis and Dunnett's test results for the SGR groups of the growth stations W, CW and CE (6 records for group)
Laboratory tests
Laboratory tests show that G. barbata was able to grow both at low nutrient concentrations (microcosms s19, s40, A, A2, C, D, G, H) and at high concentrations of nitrate nitrogen (C, C2) and phosphorus (D, E, F, I, L), while growth was impaired by ammonia nitrogen (B, B2). The thalli grew at both relatively low (s19, G) and high salinities (s40, A, A2, C, C2, D, E, F, H, I, L). The degeneration of the thallus at salinity 27 can be considered an event due to other factors, on the other hand, the test had no replicates.
The temperature was certainly an important growth factor, and the species seemed to be favoured by relatively high temperatures, at least for the population selected in the Orbetello lagoon, as shown by the 2024 test (microcosms G, H, I, L), when, after the fourth weighing, in which a plateau of thalli growth was reached for the 4 microcosms, the temperature had increased with a range between 26°C and 30°C, and there was a surge in growth in all the microcosms, making it possible to exceed, albeit slightly, the SGR value of 1% gww d-1.
The relatively high-temperature growth of the species is also confirmed by the experience of Bilajac et al.,27 which observed G. barbata collected in the Istrian Šćuza lagoon, both in the detached and in the attached form, showed remarkable resilience to climatic extremes, even prolonged temperatures of up to 32 °C and even higher for a short period. We can hypothesise that in the latitudes of the Tyrrhenian lagoons, the populations have been selected for equally important thermal stresses. Unfortunately, there are no genetic matches for the Orbetello Lagoon populations, but the endemism formation seems consistent with Riquet et al.28 who found a high genetic structure between the Adriatic Sea, Ionian Sea and Black Sea, probably the result of a gene flow limited by geographical distances, and a limited dispersal capacity of the thalli.
High (microcosms C, C2) or low and very low molar ratios (A, A2, D, E, F, G, H, I, L) did not seem to impair the growth of this species. A high N/P ratio and a preference for nitrate nitrogen, even at high concentrations, are confirmed by a laboratory growth experience conducted on thalli collected in the Gulf of Trieste, near the Grado lagoon.29 That experience compared the growth with sea water with nitrate-nitrogen dominance and P-limitation conditions with DIN/SRP molar ratios around 142, compared to water with a higher nitrate-nitrogen concentration and even higher P-limitation, but with the addition of biostimulants. In the latter microcosms, greater thalli growth was observed (SGR between 1.3 %gww d-1 and 2.7 %gww d-1 for temperatures between 10 °C and 14 °C, respectively), which also showed the development of fertile receptacles.
Lagoon test
During the study period, the two lagoon basins of Orbetello, West and East, were affected by the development of C. linum mats, where above all in the West basin, especially in the CW area, these mats were very dense, almost outcropping. This explains the high DO values, as a consequence of high photosynthetic activity, in the two stations CW and CE (Table 2). However, the much lower redox potential in CW than in CE means that there was an increase in the demolition activity of the macroalgal mat sublayer, in the first growth station. This was also confirmed by the high values of dissolved nutrients, including soluble orthophosphates, which were decidedly very high in the West basin (Table 1). Intense nutritional releases were likely occurring in the CW and W station-areas as a result of the decay of the mat layer near the bottom, which resulted in the anoxia of the sediment layer and the consequent release of ammonium30 and orthophosphate.31 The good oxidative conditions, due to photosynthetic activity along the water column and the mild spring conditions, probably favoured the nitrification of the released ammonium, while a huge amount of orthophosphate also became available as a result of the reduction of iron oxyhydroxide to ferrous ion, with the consequent release of the previously bound orthophosphates.32
SGR showed comparable behaviour between the laboratory and field test (Table 1). The value in both test groups rarely exceeded 1 %gww d-1, showing moderate growth even under favourable conditions. Relatively low values when compared to SGR estimated at around 5.5 %gww d-1 for C. linum by Sorce et al.,33 in May 2015, in the already existing mat where the CW station was located. SGR values were significantly higher in CW and W than in CE station. This could be attributed to the greater mass of Chlorophyta mats present in the West basin and the consequent greater releases of nutrients from the anoxic bottom, compared to the East basin.
Also, for G. barbata in the middle of the lagoon, the N/P molar ratio was unimportant: although nutrients were well present in both basins, in all three selected growth stations, N/P was high everywhere, in an imbalance in favour of N, with the highest value in CW (Table 1). The apparently best condition for this ratio should have been around 30, which the one in CE came close to. The value 30 was estimated by Atkinson and Smith34 as the average for the thalli of the many macroalgal species examined. This same value in the environment would favour growth conditions, although many algae developed strategies to compensate for the variable ratio conditions in the medium environment. However, it was precisely in the CE that the worst growth of G. barbata occurred.
Gongolaria barbata is a species that is frequently observed in transitional water environments; it is considered to tolerate and grow at variable salinities and relatively high temperatures. The results obtained both in the present study, in the field and in the laboratory, do not seem to contradict expectations for a species adapted to lagoon conditions: high adaptability, good responses to different salinities, temperatures and dissolved nutrients. However, it seems favoured by oxidative conditions and does not seem to tolerate pre-dystrophic, ammonium-dominated criticality. Therefore, it poorly tolerates summer criticalities, especially typical of non-tidal lagoons, when there are high ammonium nitrogen values and hydrogen sulphide releases due to the activity of sulphate-reducing bacteria. In fact, in the Orbetello lagoon, the populations of this species disappear in midsummer and this is likely attributable not to the rise in temperature as such, but to the hypoxia/anoxia produced by the accumulation of sediment organic matter and the decay of high-density macro algal mat.
G. barbata was not a particularly nutrient-demanding species in the tests conducted, being able to grow in oligotrophic, mesotrophic and hypertrophic concentrations. However, the best growth and abundance performances, almost true blooms, were observed in the Orbetello lagoon under hypertrophic conditions, with high concentrations of nitrate nitrogen and orthophosphate.
G. barbata has long been confined to the Orbetello lagoon, in oligotrophic/mesotrophic areas, close to the mouth of the lagoon with the sea, which we call area SLC1 (Figure 1). There is no certainty as to the reasons for its progressive penetration into the innermost lagoon, although it can be hypothesised that the pumping of sea-water that, proceeding from West to East, vehicles the floating thalli and has contributed over time to the distribution from the main SLC1 production area to the rest of the lagoon. Indeed, the regime of 3-5 months of pumping per year began in 1996, a period to which the beginning of the expansion of this species can be attributed.
Eutrophic conditions begin in areas more inland than the area close to SLC1, so the development of opportunistic cosmopolitan species occurs predominantly in those. G. barbata could populate the oligotrophic/mesotrophic area without competitors, but when transported to the eutrophic inner areas it competed with species with greater nutrient uptake capacities. Therefore, penetration and spread were not rapid. As nutrient availability in this lagoon increased to hypertrophy, G. barbata was able to dispose of excess nutrients and manifest vegetative blooms, as it was no longer competing for limited resources.
In the Orbetello lagoon, in recent years, the species is distributed in floating patches carried by the wind and wave motion. The greatest concentration and extensive formation of mats occur in the over stratum on the dense mats of C. linum. It cannot be ruled out that this concentration is partly produced by wave motion and that the Chlorophyta beds constitute a halt in the motion of the Ochrophyta and its accumulation. But this concentration occurs mainly in mats with a high concentration of C. linum, mats that are almost outcropping, where waves can hardly settle the species in question, except marginally. It is probable that precisely the high-density mats, by causing important nutrient releases from the sediment, as shown by the nutrient data for the waters above the C. linum mat in CW, not only support the development of the mat itself, but also favour, in their excess, a greater growth of this Ochrophyta too, which can arrive with small fragments, at least in the autumn-winter period. This species would manifest a very particular opportunistic condition. G. barbata is another native species, like the Rhodophyta S. coronopifolius10 and A. corallinum,9 which has displayed unsuspected opportunistic characteristics in the long course of eutrophic degradation of the Orbetello lagoon.
G. barbata selected in lagoon environments seems to show strong adaptability in response to the increased frequency and intensity of extreme environmental conditions, particularly water warming, as noted by Bilajac et al.27 for the Šćuza lagoon, which suggested that lagoon populations could in the future be the replacement, in restoration operations, for marine populations of this species that are in sharp decline, probably due to their inability to respond to marine warming.
None.
The authors declare that there are no conflicts of interest.

©2024 Lenzi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.