Journal of
eISSN: 2378-3184


Research Article Volume 2 Issue 4
1Department of Fisheries, University of Tehran, Iran
2Department of Biochemistry, University of Tehran, Iran
Correspondence: Mohsen Ali, Department of Marine Research, Toronto, Ontario, Canada
Received: July 10, 2015 | Published: August 29, 2015
Citation: Mirvaghefi A, Ali M, Asadi F (2015) Effects of Vitamin E, Selenium and Vitamin C on Various Biomarkers Following Oxidative Stress Caused by Diazinon Exposure in Rainbow Trout. J Aquac Mar Biol 2(4): 00035. DOI: 10.15406/jamb.2015.02.00035
Biochemical parameters are appropriate biomarkers to assess the effects of pesticides on an aquatic ecosystem. Diazinon is an organophosphate pesticide whose metabolism in fish body produces reactive oxygen species that can cause oxidative stress. In this study, Oncorhynchus mykiss fish were allocated into four treatment groups (with three replicates): control; Diazinon (0.1 mg L‑1); vitamin C (300 mg kg‑1 in diet) plus diazinon (0.1 mg L‑1) and vitamin E, selenium (selenium 0.5 & vitamin E 100 mg kg-1 in the diet) plus diazinon (0.1 mg L‑1). Blood samples were obtained after two and four weeks and superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity (TAC) and malondialdehyde (MDA) were measured. SOD activity was significantly lower in diazinon-exposed fish than in the control group. However, there were no differences between the two supplemented groups and the control group in this regard. CAT activity was significantly higher in all three diazinon-exposed groups compared to the control group. However vitamin E, selenium had the least difference with the control group. Maximum and minimum TAC were observed respectively in fish supplemented with vitamin E, selenium and those only exposed to diazinon. The diazinon group also had the highest MDA levels. The two supplemented groups and the control group had no significant differences in MDA levels. These findings highlighted the antioxidant effects of supplementation with vitamin E, selenium or vitamin C against free radical produced during the metabolism of diazinon. Meanwhile, the combination of vitamin E, selenium had higher antioxidant effects than vitamin C. Moreover, SOD, the first defensive barrier against superoxide radicals and MDA, an index of cellular damage induced by hydroxyl radicals, are the most suitable indicators to assess the effects of diazinon.
Keywords: Reactive oxygen species, Oxidative stress, Vitamin E, Selenium, Diazinon.
SOD, Superoxide Dismutase; CAT, Catalase; TAC, Total Antioxidant Capacity; MDA, Malondialdehyde; ROO, Primarily Peroxyl Radicals; GPX, Glutathione Peroxidase; ROS, Reactive Oxygen Species; GST, Glutathione-S-Transferase; GR, Glutathione Reductase; PPT, Parts Per Trillion; Ppm, Parts Per Million; RPM, Rounds Per Minute; FRAP, Ferric-Reducing Ability of Plasma; Fe-EDTA, Ferric-Ethylenediaminetetraacetic Acid; TBA, Thiobarbituric Acid; ANOVA, One-way Analysis of Variance; SD, Standard Deviation
Diazinon, O, O-diethyl O-(2-isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate as defined by the International Union of Pure and Applied Chemistry,1 is an organophosphorus pesticide, used to control various insects in both agriculture and horticulture.2 It is available in different forms, e.g. powders, emulsions and granules and in different formulations.3,4 Nowadays, organophosphorus pesticides such as diazinon are used in various agricultural activities and insects' and pest' control. They could enter surface, even underground water sources during the drainage process. The existence of diazinon, an organophosphorus pesticide, in river waters of Iran near rice paddy fields has been reported by some authors.5 Diazinon is widely used in the rice paddy fields of Gillan, Mazandaran, Golestan and other areas in Iran.6
According to a report released by journal of Ministry of Agriculture of Iran, published in 2005-2006, the annual consumption of diazinon was estimated to be 3775 tons.7 According to previous studies, the presence of this toxin in many water resources of Iran like rivers located in Mazandaran and Gholestan provinces such as Safarood, Tarkrood, Tajan, Babolrood, Haraz, Talarood, Siahrood, Ghareso, Nekarood, Ghorghanrood, Atrak and rivers located in Bushehr including Shahpoor, Dalaki, Mand and was proved and reported to be acute (over 1mg per liter) in the first days of use in agricultural activities and subacute (0.1 mg per liter) after months.8,9 Aerial transport and deposition during runoff events may be an additional source of diazinon these Both Stockton and Sacramento streams in urban streams in the cities of Sacramento and Stockton, California, USA.10 Both Stockton and Sacramento lie in storm paths that pass over agricultural areas and have accumulated a pesticide load as a result of volatilization during and after application.11 In most developing countries, one of the problems facing the aquaculture industry is the pollution of ponds and rivers with pesticides such organophosphate pesticides.12
Even more, in most tropical developing countries, the pollution of ponds and rivers with pesticides tends to be a major problem for the aquaculture industry. Chemicals such as diazinon, an organophosphate pesticide, originating from agricultural activity enter the aquatic environment through atmospheric deposition, surface run-off or leaching.13 Diazinon is a commonly used pesticide and its existence in the fresh water reservoirs of Pakistan. Diazinon exposure at low concentrations produced significant changes in some hematological parameters of Indian carp (Cirrhinus mrigala).14 Diazinon is widely used in the Mekong river delta in Vietnam. In areas where rice and fish are farmed together, for example effects of repeated exposure of diazinon in snakehead fish (Channa striata) cause significant 30% growth inhibition.15 In a similar study, Diazinon is one of the most commonly used chemicals on rice paddies, that cause prolonged negative effects in the common and commercially important snakehead fish.16
Diazinon has a half-life of about 30-39 days in waters of lakes and rivers17,18 and Diazinon breakdown is faster at warmer water temperatures, degrading 2-4 times faster in water at 21 °C compared to water at 10 °C.19 There is much evidence suggesting that high stability and density of diazinon in the aquatic environment can influence negatively the aquatic organisms in various ecosystems.20 Diazinon can be absorbed into the fish’s body through the epithelial tissue of the gills, skin and digestive system. After entering the blood, diazinon will be metabolized by liver cells.21 Metabolism of diazinon in liver microsomes of rainbow trout (Oncorhynchus mykiss) produces hydroxypyrimidine, hydroxy diazinon and hydroxymethyl diazinon. These metabolites contain a hydroxyl agent which can easily transform to a strong free radical.22,23 They also contain active polar groups such as OH- which can be disposed by participation in conjugated compounds. They may, on the other hand, turn into oxygen free radicals, e.g. hydroxyl and superoxide anion, which are capable of attacking and damaging cellular components.23 An obvious example of such damage is caused by the free radical chain reaction mechanism proceeding lipid peroxidation during which free radicals steal electrons from cell membranes.24
To overcome toxicological stress, fishes have various enzymatic defense mechanisms including the secretion of specific antioxidant defense enzymes, e.g. superoxide dismutase and catalase and formation of a cellular antioxidant defense system to scavenge free radicals.25 SOD is the main defense factor against superoxide anion radicals and is in fact considered as the first line of defense against oxidative stresses. It accelerates the transformation of superoxide anions into molecular oxygen and hydrogen peroxide.26,27 On the other hand, CAT, which is found in cells of aerobic organisms, decomposes hydrogen peroxide to water and oxygen.28 Moreover, non-enzymatic antioxidant defense mechanisms involve various chemical groups such as vitamins, carotenoids, amino acids and peptides.29,30 Vitamin E is a fat-soluble vitamin with antioxidant properties. It prevents lipid peroxidation and protects cell membranes by establishing a structure against primarily peroxyl radicals (ROO).31 In addition to vitamins, selenium can play a major role in the removal of free radicals. As glutathione peroxidase (GPX), a selenium-containing enzyme, contributes to the decomposition of hydrogen peroxide,32 selenium can be regarded as a micronutrient that indirectly protects the cells of an organism from serious damages caused by the presence of hydrogen peroxide.32 Therefore, dietary supplementation with a combination of vitamin E and selenium may protect cell membranes against lipid oxidation33,34 and exert synergistic effect in hydroperoxide detoxification.33
Another non-enzymatic antioxidant is vitamin C (ascorbic acid). Since most bony fishes lack L‑gulonolactone oxidase to convert glucose and are thus unable to synthesize vitamin C, they need to receive this vitamin from food.35,36 Intake of adequate vitamin C by rainbow trout can neutralize the revival of O2-, OH and H2O2 free radicals and prevent the damages caused by oxidative stresses.35,37 All these antioxidant factors present in the body of an organism, either intracellular enzymes or antioxidant nutritional compounds (non-enzymatic factors), are called its total antioxidant capacity (TAC).38 In other words, TAC shows how capable an organism is in removing free radicals.39
Physiological and biological changes in the bodies of various organisms, especially the fish, are appropriate biomarkers for the evaluation of environmental contaminants and environmental health of aquatic ecosystems. Such biomarkers are considered as warning signals received from living organisms to acquaint humans with the hazards of different environmental pollutants.40 Biomarkers are defined as changes in biological responses of an organism (at cellular, molecular, biochemical and physiological levels) after exposure to toxins or chemicals in the natural environment.41
Understanding biochemical mechanisms, including antioxidant defense, involved in detoxification processes, can help elucidate the required biomarkers to evaluate the damaging effects of various environmental pollutants in aquatic systems. For instance, SOD and CAT are the first line defense against reactive oxygen species (ROS). Cheung et al. assessed the suitability of numerous antioxidant parameters such as SOD, CAT, GPX, glutathione-S-transferase (GST) and glutathione reductase (GR) as biomarkers in various organisms.42 Moreover, malondialdehyde (MDA), the end product of lipid peroxidation, is directly related to cell damage during oxidative stress and can be used in evaluation of such damages.43
Since the many of the current water resources and groundwater which are widely used for aquaculture, containing also pesticides such as diazinon, this could be consequently a serious threat to fish exposed to the toxin. The main objective of this study was to reduce the adverse effects of environmental toxins in trout farming with the use of an antioxidant compound. In this respect, the present study aimed to investigate the antioxidative effects of dietary vitamin E, selenium and vitamin C in the rainbow trout exposed to subacute dosages of diazinon.
This was an in vivo study with ecotoxicological assessment.
Experimental animals and diets
A total of 180 immature rainbow trouts (mean weight: 121 ± 18 g, mean Total length: 22.9 ± 1.6 cm) were purchased from a fish farm in Karaj, Iran (35°49’N/51°1’E). The fish were transferred from a 1000 L tank into four 300 L fiberglass tanks filled with farm water and aerated with oxygen cylinders. A period of seven days was considered for fish adaptation. During the whole experiment, 10% of the total volume of tank water was changed per day. The mean temperature, pH, dissolved oxygen and water hardness during the experiment period were 12.5 ± 1.0°C, 7.8 ± 0.1, 8.0 ± 0.5 mg/L and 205 ± 16 (mg/L CaCo3), respectively. According to previous studies, the optimal levels of selenium, vitamin E and vitamin c in the diet of rainbow trout are 0.15-0.40 mg kg-1 in the diet (selenium),44 100 mg kg-1 in (diet vitamin E )45 and 300 mg kg-1 in the diet (vitamin c),37 respectively. The fishes were divided into four groups of 15 fishes each one, with three replicates. While the control group remained in unpolluted water, the other groups were exposed to 0.1 mg/L diazinon.
During the experimental trial (4 weeks) fish were hand-fed (2% of BW) twice daily (08:00 and 18:00). As basal diet used a commercially available trout fish diet (Behparvar Co. Karaj, Iran). In order to add vitamin C to the diet, 300 mg of vitamin C per kg diet carefully weighed by scale sensitive laboratories 0.0001 was dissolved in water and the pellets was sprayed. After spraying, feeds were air-dried at 25°C and kept in plastic bags in -4°C. Also, A single dose of vitamin E and selenium prepared by the Damloran Razak Pharmaceutical Company (Contains: sodium selenite 0.5 mg kg-1 in diet & vitamin E 100 mg kg-1 in the diet) was incorporated in a basal diet formulated for rainbow trout (Table 1). The dietary ingredients were blended with water to form a paste which was then passed through a meat grinder equipped with a 4.5-5 mm diet to obtain uniform pellets. Then, feeds were air-dried at 25°C and kept in plastic bags in -4°C. This diet was produced in the laboratory of the Aquatic Hygiene Department, University of Tehran.
|
Crude protein% |
Crude fat% |
Fiber% |
Phosphorus% |
Moisture% |
Ash |
Metabolize energy (Kcal/g) |
|
40.5 |
11 |
4.5 |
0.7 |
9.1 |
7.5 |
345.9 |
Table 1 Type and composition of dietary Basal diet (% dry weight)
Chemical materials and equipment
Diazinon (Partovnar® Co., Iran), in the emulation form (60%) and soluble in 40% Zylon were used in preparing the treatments. In order to produce subacute composition of diazinon (0.1 mg/L), the stock solution of 10 parts per trillion (ppt), i.e. 0.1 of its sub-lethal concentration,46 was produced.47 Encapsulated L-ascorbic acid-2-phosphate, a form of vitamin C which is stable in water, was also bought (Tiger Co., China). The chemical formula and molecular weight of vitamin C were C6H9O9P and 256.11 g/mol, respectively. A solution containing 100,000 mg/L vitamin E and 500 mg/L selenium (sodium selenite) was also prepared (Damloran pharmaceutical Co., Irana). The chemicals needed for biochemical factor measurement were Pyrogallolle solution, Tris buffer solution, hydrogen peroxide 33% (H2O2), ammounium molybdate 4%, thiobarbituric acid 0.67%, Phosphate buffered saline solution (Na2HPO), sodium benzoate, uric acid, sodium chloride (Nacl), acid acetic and butyl hydroxyl toluene. These chemicals were purchased from Sigma and Merck (Sigma, Merck Co., Germany).The spectrophotometer, Unico version UV-2100, sudatorium or Ben Murray (Shimaz Co.) and centrifuge (Genofuge M16) were used in this study.
Preparation of serum samples
Sampling was performed at the end of the second and fourth weeks. A clover powder solution of 100 parts per million (ppm) was used to anesthetize the fish during blood sampling. Blood samples were obtained from the hemal arch in the caudal peduncle using 2 mL syringes (without heparin as an anticoagulant). Five fish from each tank were randomly selected at each sampling time. The samples were transferred into 2 cc tubes (Eppendorf International, Germany) and centrifuged at 4500 rounds per minute (rpm) for 15 minutes. The isolated serums were then maintained in a freezer at -70°C until analysis.
SOD measurement
The method suggested by Marklund48 was applied to assess SOD levels in serum samples. The mentioned method measured SOD activity based on the oxidation of pyrogallol in the presence of hydrogen peroxide. Since serum levels of SOD in serum determine the reduction in pyrogallol oxidation, decreased absorption of light (420 nm) in spectrophotometry was measured. One unit of SOD activity was defined as the amount of enzyme which causes 50% inhibition of pyrogallol autoxidation.
CAT measurement
CAT activity was assessed according to the method proposed by Goth.49 At first, serum samples and hydrogen peroxide solution were mixed and maintained at room temperature for 10 minutes. Ammonium molybdate was then used to stop the oxidation process and determine the CAT activity. Goth demonstrated that higher CAT activity in serum was associated with less light absorption at 410 nm. Therefore, each unit of enzyme activity was defined as enzyme activity which could decompose 1 mm of hydrogen peroxide in one second.
TAC measurement
Ferric-reducing ability of plasma (FRAP) assay, proposed by Koracevic,50 was used to examine TAC. Accordingly, a standard solution of complex ferric-ethylenediaminetetraacetic acid (Fe-EDTA) and hydrogen peroxide produced hydroxyl radicals during Fenton reaction. The resulting reactive oxygen then caused thiobarbituric acid (TBA), a reactive acid, to be released. In this method, according to total antioxidant serum samples studied, the production of TBA was inhibited. Absorption at wavelength of 532 nm will then be reduced as a result of decreased color production.
Lipid peroxidation measurement using MDA
The method suggested by Ledwozyw et al.51 was employed to assess MDA. Hence, 1ml of the obtained serum samples was mixed with 2 ml trichloroacetic acid, TBA and hydrochloric acid under acidic conditions. The mixture was then diluted to 200 ml with distilled water and settled in a sudatorium for 30 minutes. Afterward, the tubes were transferred in vitro to be cooled. Later, the samples were centrifuged for at 300 rpm for 10 minutes and the supernatant solution was carefully isolated. The absorption rate at 535 nm was finally calculated. As the level of absorption depended on TBA inhibition by MDA in the serum, higher MDA levels caused greater TBA inhibition and less color production (absorption).
Statistical analysis
One-way analysis of variance (ANOVA) and Turkey’s tests were applied to analyze the collected data. All analyses were performed in SPSS for Windows 16.0 (SPSS Inc., Chicago, IL, USA) at a significance level of P < 0.05). Bar graphs were also drawn in Microsoft Excel 2007 to depict the mean ± standard deviation (SD).
The present study evaluated the activity of antioxidant enzymes and lipid peroxidation during oxidative stress in rainbow trout. In fact, we exposed the fish to diazinon and assessed the efficacy of vitamin C and vitamin E plus selenium in reducing its adverse effects.
Evaluation of SOD activity
The results of ANOVA for SOD levels in the second and fourth weeks, untreated fish exposed to diazinon had significantly lower SOD levels compared to the control group (P < 0.001). No significant difference in SOD level was detected between the unsupplemented fish exposed to diazinon and those supplemented with vitamin C (P = 0.117). The SOD activity levels in fish exposed to diazinon and treated with vitamin E plus selenium did not have a significant difference with the levels in the control group (P = 0.299). However, SOD increased significantly in the specimens treated with vitamin E plus selenium compared to the untreated diazinon-exposed group (P = 0.016). On the other hand, after four weeks, SOD decreased significantly in the group exposed to diazinon compared to the other groups. Meanwhile, the three groups were not significantly different in this regard (Figure 1). The results obtained after two and four weeks suggested that exposure to 0.1 ppm diazinon could decrease SOD levels in rainbow trout. Increased volume of superoxide anion radicals (O2.-) after the metabolism of diazinon might have been responsible for this finding. A previous study justified decreased SOD levels in rainbow trout 24, 48 and 72 hours after exposure to 0.5-1.0 ppm diazinon by the depletion of the enzyme following the removal of superoxide anion radicals52 (Figure 1).
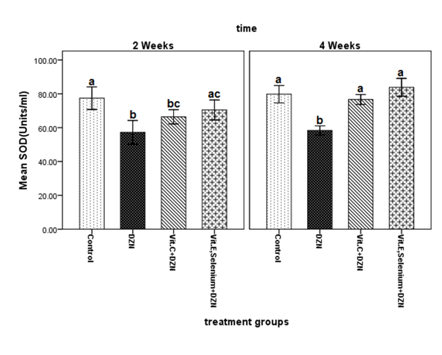
Figure 1 Changes in superoxide dismutase (SOD) activity levels in serum samples of fish exposed to diazinon (0.1 mg/L) and treated with either vitamin E, selenium or vitamin C compared to the control group after two and four weeks.
Different alphabetic letters (a, b, c, d) show significant differences (P < 0.05) and similar alphabetic letters show lack of a significant difference between groups.
DZN: Diazinon; Vit: Vitamin
Also, we could not establish significant differences between SOD levels of the control group and the diazinon-exposed fish supplemented with vitamin E plus selenium at any time. Likewise, SOD levels of the control group and the fish fed with vitamin C were not significantly different at the end of the fourth week. In the present study, SOD levels in the control group and the groups supplemented with either vitamin C (after four weeks) or vitamin E plus selenium (during the whole study) were not significantly different. This finding can be justified by antioxidant activity of the supplements (especially vitamin E plus selenium) against superoxide anion radicals produced during the metabolism of diazinon. Such an activity mimics the effects of SOD and prevents its depletion following diazinon exposure. Adding vitamin E to the diet of African catfish (Clarias gariepinus) with long-term exposure to atrazine resulted in decreased SOD levels in the liver tissue. This highlights the similar function of vitamin E and SOD in decreasing free radicals.53 Vitamin C, on the other hand, can lose one electron and reduce ROS. It can also modulate the antioxidant enzyme levels.54
Evaluation of CAT activity
At the end of the second and fourth weeks, CAT activity was significantly higher in fish exposed to diazinon than in the control group (P < 0.001) (Figure 2). CAT levels in fish treated with vitamin C increased significantly compared to the control group (P < 0.001). On the other hand, there was no significant difference between the untreated diazinon-exposed group and vitamin C supplemented fish in two stages of sampling (Figure 2). Meanwhile, the group supplemented with vitamin E plus selenium had significantly lower CAT levels compared to vitamin C-treated and untreated fish exposed to diazinon (P < 0.001). Moreover, increased CAT levels were observed in the untreated diazinon-exposed fish after both two and four weeks. This finding indicated the elevated amounts of hydrogen peroxide (H2O2) as a result of SOD activity. Research has shown 40 days of exposure to atrazine and chlorpyrifos to raise CAT activity in the liver tissues and gills of the Cyprinus carpio. As mentioned, higher levels of superoxide anion radicals following the exposure trigger the greater production of the enzyme.55 It can thus be argued that certain types of diazinon metabolism caused by the production of oxygen free radicals can ultimately lead to decreased SOD levels and increased CAT levels (Figure 2).
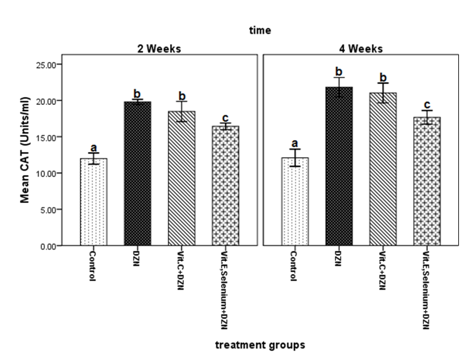
Figure 2 Changes in catalase (CAT) activity levels in serum samples of fish exposed to diazinon (0.1 mg/L) and treated with either vitamin E, selenium or vitamin C compared to the control group after two and four weeks.
Different alphabetic letters (a, b, c, d) show significant differences (P < 0.05) and similar alphabetic letters show lack of a significant difference between groups.
DZN: Diazinon; Vit: Vitamin
Also, CAT levels in fish treated with vitamin E plus selenium were significantly lower than other groups at the end of the second and fourth weeks. This can be justified by the antioxidant activity of these nutrients in the decomposition of hydrogen peroxide (which is parallel to CAT activity). GPX is an important selenium-containing antioxidant enzyme which acts similar to CAT in neutralizing free. On the other hand, two- and four-week treatment of diazinon-exposed fish with vitamin C was associated with significant increments in CAT levels (similar to untreated diazinon fish). The difference remained significant at the end of both the second and fourth weeks. This increase is probably due to the inability of vitamin C to degrade hydrogen peroxide, i.e. the increase in CAT level indicates increased amounts of hydrogen peroxide during the intracellular detoxification process. A similar study using an antioxidant compound called silymarin reported reduced CAT levels in rainbow trout exposed to diazinon. The authors explained their finding by the secretion of diazinon metabolites in presence of silymarin.56 We found comparable results in the group supplemented with vitamin E and selenium. However, introducing lycopene, a carotenoid and antioxidant, to the diet of Cyprinus carpio exposed to chlorpyrifos (an organophosphorus pesticide) could increase CAT levels.57 Therefore, the effects of lycopene and vitamin C are similar.
Evaluation of TAC
Fish exposed to diazinon had significantly lower TAC compared to the control group (P2weeks < 0.001; P4weeks = 0.033) (Figure 3). Two weeks of vitamin C supplementation significantly increased (P < 0.001) TAC in comparison with the untreated, diazinon-exposed and control groups. However, this difference did not remain significant (P = 0.055) at the end of the fourth week. On the other hand, among all groups, diazinon-exposed fish supplemented with vitamin E plus selenium had the highest TAC at the end of the second and fourth weeks (P < 0.001) (Figure 3). In the current study, TAC of diazinon-exposed fish was significantly lower than that of the control group at both sampling times. This reduction in antioxidant capacity occurred due to the process of cleaning and scavenging of free radicals by enzymatic and non-enzymatic antioxidant defense systems. A previous study indicated that subacute diazinon affected liver hepatocytes in rainbow trout and resulted in decreased TAC. The researchers introduced excessive production of free radicals during the metabolism of diazinon in the liver (the detoxification process) as the main reason for this finding.5 Monterio et al.58 reported oxidative processes which increased ROS to contribute to reduce TAC (Figure 3).

Figure 3 Changes in total antioxidant (TAC) activity levels in serum samples of fish exposed to diazinon (0.1 mg/L) and treated with either vitamin E, selenium or vitamin C compared to the control group after two and four weeks.
Different alphabetic letters (a, b, c, d) show significant differences (P < 0.05) and similar alphabetic letters show lack of a significant difference between groups.
DZN: Diazinon; Vit: Vitamin
We also detected the diazinon-exposed fish treated with vitamin E plus selenium to have significantly higher TAC compared to other groups. This finding reflects the higher antioxidant capacity of this combination in comparison with vitamin C. In addition, adding vitamin C to the diet of the diazinon-exposed fish significantly increased TAC levels compared to the untreated diazinon-exposed fish and the control group. Vitamin E, ascorbic acid (vitamin C), uric acid and glutathione comprise about 70% of the total antioxidant activity. Winston et al. suggested vitamins E and C to make up a great share of the TAC59 Research has also revealed the association between increased TAC and enhanced ability of an organism to neutralize and remove free radicals.60 As vitamin E and selenium are critical components of the antioxidant defense and major determinants of TAC, the highest levels of TAC in fish supplemented with these two nutrients in the present study seem logical.
Evaluation of MDA levels
At the end of the second and fourth weeks, the diazinon-exposed fish had significantly higher MDA levels compared to the control group (P < 0.001) (Figure 4). MDA levels in the fish fed with vitamin C were significantly higher than that in the control group only at the end of the second week (P = 0.016). No such a difference was detected after four weeks (P = 0.427). However, supplementation with vitamin E plus selenium prevented cellular destruction caused by diazinon exposure and maintained MDA levels close to the levels in the control group (P2weeks = 0.797; P4weeks = 0.990) (Figure 4).

Figure 4 Changes in malondialdehyde (MDA) activity levels in serum samples of fish exposed to diazinon (0.1 mg/L) and treated with either vitamin E, selenium or vitamin C compared to the control group after two and four weeks.
Different alphabetic letters (a, b, c, d) show significant differences (P < 0.05) and similar alphabetic letters show lack of a significant difference between groups.
DZN: Diazinon; Vit: Vitamin
Lipid peroxidation evaluations in the present research showed that after two and four weeks, MDA levels were significantly higher in the untreated diazinon-exposed group than in the control group. Such increments reflect the higher production of hydroxyl radicals (OH-) subsequent to exposure to the poison. Hydroxyl radicals are oxidizers with a key role in the initiation of lipid oxidation processes. Increased MDA levels were also confirmed in a study on the effects of 15 and 30 days of exposure to 0.1 mg diazinon on muscle tissues and kidneys of Oreochromis niloticus.61 In general, decomposition of diazinon can generate oxygen free radicals, such as superoxide anion radical and strong hydroxyl ions, which in turn raise MDA levels. This increase may be due to the decomposition membrane lipids during exposure to the mentioned toxic metabolites.62,63 In the current study, MDA levels in the fish supplemented with vitamin E plus selenium and the control group were not significantly different. However, adding vitamin C to the diet of diazinon-exposed fish significantly increased MDA levels (compared to the control group) at the end of the second week. Nevertheless, such a difference was not present at the end of the fourth week. According to available literature, vitamin E is a strong antioxidant which can prevent lipid peroxidation caused by the formation of lipid hydroperoxides.33. Selenium is also an important part of GPX and is responsible for reducing oxygen free radicals and interrupt lipid oxidation.64 Using dietary vitamin E reduced lipid peroxidation in liver tissues of female African catfish (Clarias gariepinus) dealing with chronic atrazine exposure.53
The results of our work show that induction of subacute dose of diazinon (0.1 mg L‑1) caused significant changes in the level of super oxide dismotase and total antioxidant capacity. Also, exposure to diazinon increased the level of catalase activity and lipid peroxidation throughout the period the experiment. Application of dietary vitamin E, selenium and vitamin C, as a factor enhancing the antioxidant capacity of the cell, modulated and adjusted SOD, CAT enzyme activity levels and increased the total antioxidant capacity of the cells. Furthermore, vitamin E, selenium and vitamin C cause reduced lipid peroxidation. In addition, the combination of vitamin E, selenium had higher antioxidant effects than vitamin C. In other words, this level of vitamin C was less effective than vitamin E, selenium. Considering the possible presence of diazinon in aquatic ecosystems and its damaging effects, the use of vitamin E and selenium can be enhance non-enzymatic antioxidant defense and adjust the levels of SOD and CAT to their normal values. Such an intervention can also increase TAC in fish. Also, MDA measurements in the present study showed the efficacy of vitamin E and selenium in preventing lipid peroxidation. Vitamin C was also partially beneficial in the adjustment of SOD activities. Moreover, vitamin C was less effective than vitamin E plus selenium in improving TAC and inhibiting lipid peroxidation.
On the other hand among all measured biochemical factors, SOD is the first specific antioxidant defense barrier against superoxide anion radicals. In addition, it is more sensitive than CAT to exposure to diazinon. Therefore, SOD seems an appropriate biomarker for monitoring aquatic ecosystems. While evaluating total antioxidant levels (TAC) is crucial to the general investigation of oxidative events in a biological system, the interpretation of changes in biochemical factors in complex ecological levels is difficult due to several factors. MDA is an indicator of lipid peroxidation (caused by the exposure of cell membranes to free hydroxyl radicals produced during the metabolism of diazinon). As it reflects the level of damage in aquatic organisms, it has been suggested as a reliable biomarker to assess the effects of environmental pollutants. Our study clearly demonstrated that introducing a combination of vitamin E and selenium into the diet of rainbow trout, especially during the agricultural season when the presence of diazinon in waters is more probable, could enhance the fish’s antioxidant defense. In conclusion, considering the studies conducted to in examine the presence of agricultural contaminants and pesticides in domestic water sources and their destructive effects on various species of fish, the use of suitable materials with antioxidant characteristics in the formulation of proper diets for the different species of fish, in order to increase their antioxidant defense, seems necessary.
The present study was conducted using the equipment available in the Faculty of Natural Resources of Tehran University (Iran). We wish to acknowledge all those who supported us in conducting this research.
None.

©2015 Mirvaghefi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.