Journal of
eISSN: 2378-3184


Research Article Volume 3 Issue 2
Department of Marine Biology, Ecological Foundation, Spain
Correspondence: Laura Del R
Received: July 21, 2015 | Published: December 4, 2015
Citation: Torres LDR, Galván V (2015) Diadema antillarum Population Status Assessment in Dominican Republic 30 Years after the Mass Mortality Event. J Aquac Mar Biol 3(3): 00066. DOI: 10.15406/jamb.2015.03.00066
The long-spined sea urchin Diadema antillarum plays an important role in the coral reef community dynamics and geomorphology of the Caribbean. A dramatic die-off event in 1983-84 reduced its population by as much as 95-99% throughout the Caribbean resulting in dramatic changes in the benthic community. This study aims to report population density; bathymetric distribution and population size structure of this sea urchin in four localities of Dominican Republic: Bayahibe, Punta Cana, Samana and Sosua. For this study, 15 m2 transects were conducted using a 0.5 x 0.5 (0.25) m2 quadrants at Acropora cervicornis restored reef sites. Transects were laid in and out the restored plots. The benthic cover was also estimated and classified in 5 main categories: macroalgae, coralline algae, corals, sand/rock/rubble and sponges. The mean density of D. antillarum obtained for the entire study was 0.89±0.11 Ind·m-2 (Mean ± SE, n=48). The distribution of this sea urchin was mostly limited to depths comprised from 1-10 m of the sublittoral; no significant differences were observed in the vertical distribution by age. The mean benthic cover found for the four localities was: Coral 6.75%, Macroalgae 22.66%, Coralline Algae 24.99%, Sand/Ruble/Rock 41.75% and Sponges 2.68%; although substrate communities vary significantly at different sites even within same localities, depending on multiple factors affecting the area. A discrete slow patchy recovery of D. antillarum has been observed in Dominican Republic.
Keywords:Diadema antillarum, Population, Status assessment, Dominican republic, Mass, Mortality event, Acropora cervicornis, Caribbean, Macroalgae, Coral reef, Bayahibe, Punta cana, Samana, Sosua, Diadema, Estuarine, Sponges, Sediments
Coral reef ecosystems provide valuable social services and play an important role in the economy along the Caribbean. These ecosystems are of high complexity, understanding and assessing biodiversity is essential for coral reef communities and for future management improvements. The long spine sea urchin Diadema antillarum is a key species found in shallow waters of the Eastern and Western Atlantic Ocean and Caribbean Sea. Its feeding habits deeply influence the geomorphology and dynamics of the communities related to coral reef ecosystems such as algal cover and coral diversity. D. antillarum competes with other sea urchins and herbivorous fish and can be found grazing in coral reefs, rocky shores and sandy bottoms and are often associated with causing bare areas in sea-grass beds.1,2 They are also considered principal agents of bioerosion on reefs.3-5 Although D. antillarum feeding habits have been related to a preference for algae, is still considered an omnivore organism ingesting other sessile invertebrates6 including corals, especially Acropora cervicornis when present.7 The long spines of D. antillarum alsoserve as refuge for larvae for a variety of species such as the Caribbean spiny lobster Panulirus argus, several species of fishes andinvertebrates like copepods, mysids, shrimps and crabs (Figure 1).
Before the 1980’s D. antillarum was widely distributed throughout the Caribbean and West Atlantic, from the Gulf of Mexico and Bahamas to Surinam;2 however, between 1983-1984 a mass mortality event occurred, caused by a yet unidentified water-borne highly specific and virulent pathogen presumably transported by surface currents. This mass mortality event reduced D.antillarum population by as much as 99%.1,8-11
In the Dominican Republic, studies to evaluate current D. antillarum population densities were only recently implemented and have been of limited scope and reach. As a result, information gaps still need to be addressed. Only 3 previous articles were found in the Dominican Republic referring to this echinoid reporting Diadema antillarum abundance dating from 2003, 2007 and 2013. Although these previous studies suggest a modest increase, 0.11 to 0.28 individuals/m2, from 2003 to 2013 respectively,12 no literature previous to the die-off event has been found for a baseline comparison.
The main goals of this study are to continue monitoring, expand on previous studies and assess the current Diadema antillarum population density, size structure and bathymetric distribution along the Dominican Republic neritic zone, 30 years after the massive die-off. An additional objective was to obtain a brief characterization of the substrate and see if changes in the benthic cover can be related to D. antillarum population density increases.
Study area
Since 2004 the Puntacana Ecological Foundation has a big on-going coral gardening and restoration project with over 9 coral nurseries in a similar number of coastal communities; these 9 nurseries are harvested for coral fragments and out planted onto natural coral reefs. From these coastal communities, a total of four were selected based on logistical opportunities and included: Punta Cana, Bayahibe, Samana and Sosua. All sites selected included an Acropora cervicornis restoration project with at least 6 months of establishment.
To assess the D. antillarum population density, in situ transect surveys were carried out expanding on the procedures from those described in the technical report presented to the Puntacana Ecological Foundation by Gloeckler.12 The transect surveys were conducted haphazardly in a combination of out planted reef areas and natural reefs, to obtain a better understanding of the roles of different components of the reef ecosystem. Expansions to the established procedures included discriminating juvenile from mature individuals, categorizing individuals into class sizes and tracking individuals by depth (see data analysis section for detailed information).
The Punta Cana and Bayahibe sites were previously surveyed (2013) both inside and outside of the out-planted plots; here the same previous sites were surveyed using the reported coordinates as a follow up and incorporated the additional survey parameters. The present study was performed from August 2014 through January 2015. The surveyed transects were registered by GPS (GPS map 60Cx) and the coordinates used for mapping the surveyed areas. The study area encompassed the seabed of the continental shelf from 0-15 m depth regardless of the nature of the substrate, as long spine sea urchins are also found in sandy bottoms and Thalassia mounds2 where there is some shelter nearby (personal observation) (Figure 2).
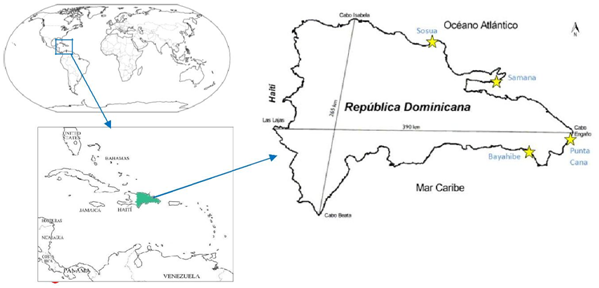
Figure 2 Dominican Republic map showing the selected study areas: Bayahibe, Punta Cana, Samana and Sosua).
Bayahibe: Bayahibe is located in the Southeastern end of the Dominican Republic, in the province of La Altagracia, on the leeward side, protected by a land mass of Pleistocene and recent reef terraces.13 Located downstream of oceanic currents, with an East to West general current all year-round and receives minimal river influence. The reef orientation is east-west, perpendicular to the shore, with low relief and spur and groove communities. The Caribbean current dominates in the coastal waters of Bayahibe, which is also influenced by the action of NE trade winds, dominating most of the year. The surveys were conducted at different localities from remote areas and low population to towns facing major resorts (Figure 3) (Table 1).
|
Bayahibe |
Latitude |
Longitude |
|
Magallan Shallow |
N 18°22'6.24" |
W 68°50'42.60" |
|
Magallan Deep |
N 18°21'39.50" |
W 68°50'20.30" |
|
Magallan Beach (MP1) |
N 18°21'49.15" |
W 68°50'29.32" |
|
Magallan Beach (MP2) |
N 18°21'48.87" |
W 68°50'28.19" |
|
Magallan Beach (MP3) |
N 18°21'48.47" |
W 68°50'27.03" |
|
PEPITO1 |
N 18°20'44.30" |
W 68°49'56.42" |
|
PEPITO2 |
N 18°20'40.13" |
W 68°49'50.99" |
Table 1 Coordinates of the sites where the transects were conducted in Bayahibe: Magallan Deep (n=7), Magallan Shallow (n=4), Magallan Beach (n=3), PEPITO1 (n=2) and PEPITO2 (n=3)
Punta Cana: Punta Cana is located on the easternmost part of the Dominican Republic and influenced by the Mona Passage that connects the Atlantic Ocean with the Caribbean Sea. Tidal currents set generally S and N through Canal de la Mona. Non tidal flows vary depending to a great extent upon the velocity and the direction of the wind, combined with the tidal current. A pronounced NNW non-tidal current along shore is set almost year round, except during the summer months where it tends to slacken, a strong countercurrent sets E off the S of la Hispaniola that occasionally induces a N set in the passage. The tidal currents also set with considerable velocity, especially near the shores of Cabo Engaño, with ebb currents setting SW, although reversed currents have also been reported (NOAA).
The coast is characterized by a patchy fringing reef system with spur and groove reef formations; the reef rises up running perpendicular to shore. Breakers marking the reef crest are located within a kilometer offshore. Depth in the lagoon between the breakers and shore varies from 0.5-4 meters. The shoreline is primarily formed by sandy beaches with occasional areas of rocky shore.14. This reef is exposed to high wave action, strong currents and heavy swells with a great dynamics of the fine sandy sediments (Figure 4) (Table 2).
|
Punta Cana |
Latitude |
Longitude |
Reef Type |
|
Aquarium |
N 18˚32’20.7" |
W 068˚20’50.6" |
Back Reef |
|
OP29 |
N 18°30'34.8" |
W 068°21'36.8" |
Fore Reef |
|
OP30 |
N 18°32,06.0'' |
W 068°20'47.8'' |
Fore Reef |
|
OP19 |
N 18°30’48.3” |
W 068°21’28.4” |
Fore Reef |
|
OP4 |
N 18°27’44.6" |
W 068°23’28.1” |
Fore Reef |
|
OP8 |
N 18°28'22.9" |
W 068° 23'02.9" |
Fore Reef |
|
DR0102 |
N 18°31'46.0" |
W 068° 21'30.0" |
Back Reef |
|
DR0501 |
N 18°33'19.0" |
W 068°20'33.0" |
Back Reef |
|
OP35 |
N 18°31'04.9" |
W 068°21'20.2" |
Fore Reef |
|
Ext OP21 |
N 18°32,01.2'' |
W 068°20'58.5'' |
Fore Reef |
Table 2 Transect surveyed coordinates for Punta Cana
Samana: The study area in the Samana Bay included the town of Samana and “Los Cacaos”, located approximately 15 minutes East of the town of Samana. This region of the Dominican Republic is very humid. The Samana Bay is influenced by the discharge of several rivers, carrying an important amount of sediments creating a seabed composed of fine estuarine sediments of terrigenous origin, probably devoid of macro-vegetation. The Yuna river system discharges to the west in Samana bay and together with the “Los Haitises” and the “Sabana de la Mar” watersheds, they form the largest estuarine system of the Caribbean islands. The waters in the entire region are generally murky due to the high loads of sediments, limiting coral growth.15
The physico-chemical characterization of this area shows significant fluctuations in all the physico-chemical water parameters, high percentages of silt and clay sediments and high concentrations of organic matter. The physical and chemical processes that occur when freshwater gets in contact with salt water, make a great amount of sediments associated with organic matter to precipitate.15 An extended area of red mangroove forests on the northern coast of the Bay has also been reported, characteristic of estuarine ecosystems (Figure 5) (Table 3).
|
Samana |
Latitude |
Longitude |
|
SMN 1 |
N 19°10'09.6" |
W 069° 16'17.7" |
|
SMN 2 |
N 19°10'11.4" |
W 069° 16'12.9" |
|
SMN 3 |
N 19°10'3.0" |
W 069° 16'17.3" |
|
SMN 4 |
N 19°10'5.7" |
W 069° 16'15.7" |
|
SMN 5 |
N 19°11'0.2" |
W 069° 15'23.8" |
|
SMN 6 |
N 19°11'44.9" |
W 069° 19'43.6" |
|
SMN 7 |
N 19°11'42.9" |
W 069° 19'45.2" |
Table 3 Transect surveyed coordinates for Samana.
Sosua: The Sosua region, located at northern coast of Dominican Republic, extends from east to west along 72 miles of the Atlantic coastline integrating a complex geosystem mosaic of plains and wetlands, fluviomarine valleys and river heights with erosive and erosive-denudation low mountains. The sublittoral geomorphology presents a rocky conglomerate similar to a coralline barrier 200 m away from the coastline, protecting the beach development. The sublittoral geomorphology is mainly formed by sandy bottoms with or without sea-grass and macroalgae with shallow coralline rocky patches with sandy channels.16 At low tide the average water flow matches with the wind westbound, but at high tide an eastward strong current can occur. This implies that under certain meteorological and oceanographic circumstances, the region may receive the influence of the Sosua River.
Recent studies showed a low biodiversity in the region, some of the impacts that can influence on the marine ecosystem are polluted waters coming from the river run off and overfishing17 (Figure 6) (Table 4).
|
Sosua |
Latitude |
Longitude |
|
SS 1 (3 Rocks) |
N 19°46'23.44" |
W 70°30'53.28" |
|
SS 2 (3 Rocks) |
N 19°46'22.56" |
W 70°30'53.52" |
|
SS 4 (3 Rocks) |
N 19°46'22.07" |
W 70°30'54.39" |
|
SS 5 (3 Rocks) |
N 19°46'21.64" |
W 70°30'54.78" |
|
SS 3 (Sosua OP1) |
N 19°45'28.40 |
W 70°31'8.40 |
Table 4 Transects surveyed coordinates for Sosua
Sampling and data analysis
Diadema antillarumpopulation density and size structure: The abundance and size of the individuals was estimated visually in situ, using the band transect method. A 15 m x 1 m (15 m2) transect was laid haphazardly at each of the survey sites. The surveyor swam along the transect counting all the D. antillarum individuals in the 15 m2 transect area, checking all the crevices and holes of the reef structure and measuring their test size using a ruler. It was unavoidable that in some cases an estimation of the test diameter was taken due to de difficulty in approaching the organism, not only because of the long spines but also sometimes the inaccessibility of the crevices where they were hiding. At sites where Acropora cervicornishad been transplanted back onto the reef as part of a restoration projectsurveys were carried out inside and outside of the restored plots. At all other locations, only one survey per site was performed.
To assess the population size structure, individuals were quantified and classified into one of three different size ranges, based in previous literature.18-21 Size ranges wereselected taking into account that most of the Diadema antillarum individuals develop gonads at a test diameter of approximately 4-5 cm.20 A newly settled D. antillarumranges from 0.75-0.84 mm in test diameter,22 individualsup to 3 cm (TD) were count as successful recruits.
|
Class 1 |
<3 cm |
Recruits: Number of juveniles that succeeded the larval stage |
|
Class 2 |
3-6 cm |
Number of individuals developing gonads |
|
Class 3 |
>6 cm |
Adults: Ensuring gonad development (breeding population) |
This classification system allows for quantification of the number of individuals at each important sea urchin life stage and facilitates data collection while diving.
Bathymetric segregation: A Mares Puk diving computer was used to record depth, with reef sites being divided into depth intervals of: 0-3 m, 4-6 m, 7-10 and > 10 m, to test if densities of D. antillarumvary along depth gradient.6,20,23,24
Benthic composition surveys: Benthic composition was surveyed by placing a 0.5 m x 0.5 m (0.25 m2) quadrant over every other half meter alternating to either the left or right of the 15 m long belt transects and taking digital photographs of the quadrant. A total of 15 quadrant photos per transect were taken using a Canon Power Shot A620 camera. The images were uploaded into the computer and analyzed to estimate percentage cover of the substrate with Coral Point Count with Excel Extensions software (CPCe) with 100 randomly superimposed points; each of these points was then classified into one of six categories: coral, coralline algae, macroalgae, sponges, sediment or other. The substrate classified as “sediments” included rubble, sand and rocky substrates (Table 5).
|
Locality |
Number |
Surveyed |
Processed |
Benthic cover area |
|
transects |
area (m2) |
images |
analyzed (m2) |
|
|
Bayahibe |
19 |
285 |
194 |
48,5 |
|
Punta Cana |
16 |
240 |
155 |
38,75 |
|
Sosua |
6 |
90 |
79 |
19,75 |
|
Samana |
7 |
105 |
88 |
22 |
|
Total DR |
48 |
720 |
516 |
129 |
Table 5 Benthic Composition Surveys
Population density
The mean density of Diadema antillarum for the four communities surveyed was 0.89±0.11 Ind·m-2 (Mean ± SE, n=48), ranging from 0 to 11.67 Ind·m-2. The total mean density at each of the four surveyed localities was highest in Sosua (1.98 Ind·m-2), followed by Bayahibe with 0.82 Ind·m-2, Samana (0.44 Ind·m-2) and Punta Cana (0.30 Ind·m-2).
In Punta Cana, previous studies by Gloeckler12 obtained D. antillarum densities of 0.11 and 0.28 Ind·m-2 respectively; 2014 surveys showed a slow but steady increase of the population of approximately 7% to 0.30 ind·m-2, compared to previous years. In Bayahibe, density increased 1.8 fold this past year from 0.45 Ind·m-212 to 0.82 Ind·m-2 in 2014. One of the transects at Sosua was conducted on an isolated rock surrounded by sand that resulted in a significant number of urchins and a density of 11.67 Ind·m-2, this inflated the overall density for the Sosua locality. The rest of the transects at this locality had a density ranging from 0 to 0.20 Ind·m-2 and the substrate was composed of sandy bottoms devoid of refuge structures (Figure 7).
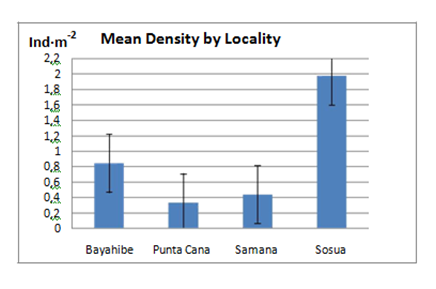
Figure 7 Total Diadema antillarum abundance in each of the 4 localities sampled showing Standard Error bars (±SE).
Population size structure
In general the larger size classes (Class 2 and 3) are present and dominate in terms of total abundance (Figure 8). For Sosua the average population density of adult D. antillarumwas 5 times greater than the average for the rest of the localities. Sosua alsohad the highest number of recruits (0.20 Ind·m-2) and a maximum density value for the adult group (breeding population) of 1.56 Ind·m-2 compared with the other locations. The lower class size range (Class 1) is also present in all locations, but to a lesser extent, with the lowest recruitment found in Punta Cana (0.05 Ind·m-2). Punta Cana has the lowest density values for all three class sizes from all locations surveyed. Bayahibe has the highest abundance of individuals in class 2 (3-6 cm) with a density of 0.42 Ind·m-2, a low recruit class 1, with a 0.08 Ind·m-2 and a 0.34 Ind·m-2 size Class 3. At Samana, in general, the abundance of D. antillarum population is low, possibly due to the high sedimentation on that area, but has a higher density of recruits compared to Bayahibe and Punta Cana.

Figure 8 Average values of Diadema antillarum abundance (Ind·m-2) for the different size ranges (cm): Class 1: <3 cm, Class 2: 3-6 cm, Class 3: >6 cm. 8.1) Total Mean Density by Size 8.2) Mean density at each locality.
Bathymetric segregation
A clear vertical pattern distribution was observed for most sites, with a notably increase of D. antillarum density with increasing depth up to 7 m; most of the D. antillarum population were found in the sublittoral environment at depths ranging from 1 to 7 m. At depths >7 m D. antillarum presence decreased and was almost absent for depths >10 m. Sosua exhibited a contrasting distribution pattern compared to all other sites with the highest density of D. antillarum (2.62 Ind·m-2) occurring at shallow depths (0-3 m). Minimum values were observed for the rest of the depth intervals at this locality, 0-0.02 Ind·m-2 for size class 2 and 3 respectively. Bayahibe D. antillarum population increased with depth from 0.51 Ind·m-2 in shallow waters (0-3 m) to 1.38 Ind·m-2 at (6-10 m) depth, for depths >10 m significantly decreased to 0.01 Ind·m-2 (Figure 9).
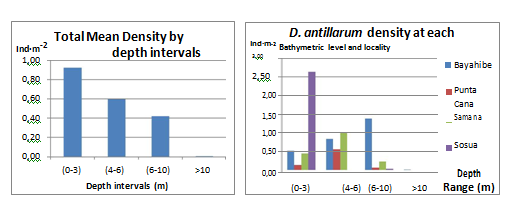
Figure 9 Diadema antillarum frequency distribution (Ind·m-2) for the different bathymetric levels: Total mean densities (left) and at the 4 different localities surveyed (right).
Bathymetric distribution for the different size ranges
No clear pattern was detected in the vertical distribution for Individuals of different age. Overall, the adult or breeding population (>6 cm) clearly decreased its abundance with increasing depth with a more clear pattern for its distribution. The intermediate size range (3-6 cm) was more evenly distributed along the sea urchin zone (1-10 m). The juveniles (<3 cm) were more abundant at intermediate depths (4-6m), increasing from the shallowest depth range (0-3 m) to a maximum abundance in the intermediate depth interval (4-6 m) and significantly decreased at depths from 6-10 m and deeper. Each community surveyed showed different patterns for the Diadema distribution, hence, supporting the hypothesis that factors affecting their distribution appear to be more related to the turbulence of water, availability of shelter structures and the number of predators or competitors for resources in the area (Figure 10). Bayahibe showed a clear pattern for all three size classes, increasing abundance with increasing depth up to 10 m, no individuals for depths deeper than 10 m. Class 2 and 3 are significantly more abundant than Class 1.The density values for Class 2 increased from 0.73-1.93 ind·m-2 and then dropped to 0.01 ind·m-2 below 10 m of depth; Class 3 densities increased from 0.65-0.93 ind·m-2 and was absent below 10 m (Figure 11).
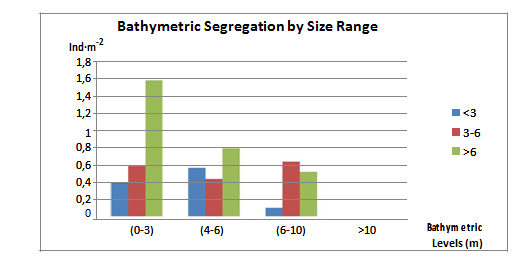
Figure 10 Diadema antillarum density (Ind·m-2) (average values±SE) for the different bathymetric levels (m) and size ranges: Class 1: <3 cm, Class 2: 3-6 cm, Class 3: >6 cm of test diameter (spines not measured).
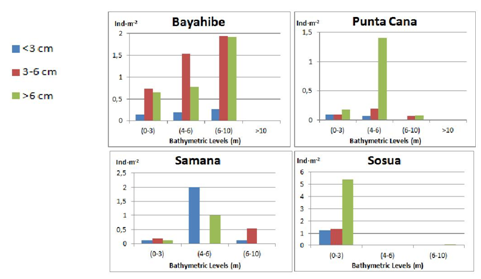
Figure 11 Diadema antillarum total mean densities (Ind·m-2) for the different bathymetric levels (m) and size ranges: Class 1: <3 cm, Class 2: 3-6 cm, Class 3: >6 cm (test diameter without spines: legend to the left) at each locality.
The results observed on the rest of localities do not show a clear pattern. Punta Cana shows a low abundance in general for the three size classes with highlighting peak (1.40 Ind·m-2) of Class 2 individuals at intermediate depths (4-6 m). Samana had a greater density of juveniles (2 Ind·m-2) at the intermediate depth interval (4-6 m) than all other sites. At Sosua the mean density of Diadema was found to be exceptionally high at shallow waters due to the concentration of this sea urchin at an island-rock that resulted on average values, for all size classes (1-3), much higher in the shallower depth interval (0-3 m): Class 1 (0.39 Ind·m-2), Class 2 (0.59 Ind·m-2) and Class 3 (1.58 Ind·m-2), Diadema was almost absent for the rest of transects.
Benthic composition
To determine benthic composition 48 transects were performed; a total of 516 photos were processed with the Coral Point Count with Excel Extensions software including: 194 images for Bayahibe, 155 for Punta Cana, 88 for Samana and 79 for Sosua. The results obtained showed similar values for macroalgal cover amongst all four sites with Sosua having the lowest macroalgal cover (19.08%), followed by Punta Cana (23.48%), Samana (23.40%) and Bayahibe (24.69%). Corals and sponges were notably more abundant at Bayahibe and Punta Cana with values of 13.54% and 7.02% respectively.
The northern regions (Samana and Sosua) presented higher percentage of sediments, 72.88% and 70.10% respectively; while Bayahibe and Punta Cana had 45.86% and 47.11%. The Sponge coverage found at Samana (1.17%), Sosua (2.78%) and Punta Cana (1.59%) was very low. Coralline algae cover was much lower at the northern localities; it was completely absent in Samana, while Sosua had a value of 1.16%. Greater values were observed at Bayahibe 8.4% and Punta Cana 18.65%. The mean benthic cover for the Dominican Republic, based on these four localities was: 6.75% Coral; 22.66% Algae; 7.06% Coralline Algae; 58.99% Sediments and 3.14% Sponges (Figure 12).
Population density
The long spine sea urchin Diadema antillarum was present at all the locations surveyed in the Dominican Republic. The average value for the Diadema antillarum density, based on the 4 localities surveyed (Bayahibe, Punta Cana, Samana and Sosua) was 0.89±0.11 Ind·m-2 (Mean ± SE). Although densities of D. antillarum prior to mass mortality are unknown for the Dominican Republic; overall, the population densities were low across the DR, compared to earlier studies from neighboring islands where densities ranged from 0.83-1.55 Ind·m-2 in Puerto Rico in 2004 to 1.50 and 2 Ind·m-2 in Dominica, 2001 and 2004 respectively25 to 5-12 Ind·m-2 in 200026 and 2.77 Ind·m-2 (2010) in Jamaica.27 The lack of historical density values makes it difficult to evaluate the status of the current population but an apparent yet slow recovery over the last 11 years continues to be observed.
At the individual community level Punta Cana D. antillarum populations continue to increase but at a much slow pace than Bayahibe. The faster increase of D. antillarum in this area could be explained by the more favorable conditions: less surge action, weaker currents and less predatory pressure (removal of sea urchin predators due to overfishing), as previously speculated by Ogden et al.28 Hay,29 Carpenter,6 Debrot & Nagelkerken30 and Goldber.5 Bayahibe is located downstream of the Caribbean current and sheltered by La Altagracia Southern projection and Isla Saona, forming a screen to the SE, NE and E winds, with a moderate intensity littoral current from East to West, suchlike a leeward bay; this geomorphology might contribute in increase recovery rates or recruitment levels.
The low densities of Diadema observed in Punta Cana can also be explained by the highest wave action and strong currents at that locality; its long spines conveniently designed to minimize the risk of being attacked by potential predators are at the same time an inadequate mechanical design for turbulent environments,31 its morphological design does not allow a large adhesive area to attach, being considered weakly resistant to wave exposure.31,32 Samana is located at the Samana Bay, influenced by the discharge of the Yuna and Barracote rivers, with great physico-chemical variations in water characteristics, a predominant muddy substrate and fewer coral and rocky patchy substrates.15 The highest D. antillarum abundance observed at Samana was found at SMN5 (1.13 Ind·m-2), with a rocky substrate presenting abundant crevices offering higher shelter opportunity, this transect presented the highest number of recruits, coinciding with the highest number of adults, suggesting that recruits aggregation may be influenced by adult aggregation as previously observed by Hunte & Younglao;10 SMN5 was conducted at “Playa Los Cacaos”, the geomorphology of the coastline may be implicated in the retention of larval pools entering with tide flows. This was also encountered at Sosua, where the transect conducted at the rocky island (SS1) seemed to be an “oasis” for D. antillarum, showing the highest abundance found in this study (11.67 Ind·m-2), this behavior has previously been observed, where organisms settling from the water column take advantage of the single available shelter; although, the fact that the other 2 rocky islands nearby didn’t have such an abundance (0-0.20 Ind·m-2) suggests that the presence of adults segregation triggers larval settlement of this echinoid, either by providing suitable substratum (cryptic surfaces free from algae), inducing settlement by chemical signals or the shield provided by the near adult conspecifics.32,33 The high densities found at this one transect could also be explained by the distance to reach another source of food.
The findings of this study are consistent with the results from previous literature where highest abundances of this echinoid are found at leeward wave sheltered areas30,32 and at small bays and other inland enclosures where tidal residual currents are pervasive and shoreward advection may allow larval pools to accumulate.30 No previous data has been reported for Samana and Sosua making their continued monitoring as part of an integral species recovery monitoring is recommended. Furthermore, expanding the number of localities with different habitat conditions can broaden our understanding of the factors affecting the population dynamics, growth and its impact on the coral reef communities. The results obtained in this study showed no differences in Diadema abundances in and out the Acropora cervicornis restored plots. All the surveys were conducted during daylight and although extra effort was put on to check all holes and crevices in complex substrate transects the results obtained can be conservative values, adding some night dives could increase these results, as some individuals hide deeply in the reef structure and have a nocturnal feeding behavior.5,18
Population size structure
In the surveyed regions from the Dominican Republic, populations of Diadema antillarumshowed a size distribution with predominant medium to large sizes (4 to 9cm of test diameter) and very few small (<3 cm) individuals. No previous data was found for size frequency distribution for the Dominican Republic, although previous studies in Puerto Rico report a similar size distribution.2,20,32,34 The low densities observed for size Class 1 (<3 cm) might be an indicative of a low level of recruitment or juvenile survival or either a high mortality of juveniles.20 Hydrodynamics, substrate complexity and local adult densities seem to play an important role in larvae settlement of this echinoid as has been observed in other invertebrate larvae.35
antillarumhas a planktotrophic echinopluteus larva with a planktonic phase rangingfrom 36 days4 to 50-90 days36 to metamorphose, varying this time depending on exogenous resources.22 During this time D. antillarumis exposed to predators and transported by currents offshore, so it may be increasing mortality rate by predation and the potential of dispersal reducing larval chances to return to the coastal areas of the Dominican Republic, dispersal role might be an important factor diluting its population.35 D. antillarumlarvae are much larger than most echinoid larvae;4 its longspines may avoid some predators by being harder to swallow, but this mechanism may not be an effective defense against other predators such as hydromedusae, ctenophores, euphausids and chaetognaths43,22,37 while increasing drag,4,38 dispersal distances from source populations to sites of settlement and recruitment are thought to be on the order of tens to hundreds of km.10,39 Larvae pools may passively accumulate and drift under the influence of hydrodynamical processes operating at large scales (tens of meters to tens of kilometers), while active substratum selection may occur at much smaller scales (centimeters to meters),32 with a clear preference of Diadema larvae for hard substratum free of non-calcareous algae17,32 that the adult conspecifics may be promoting. The way by which competent larvae leave surface waters to settle on the bottom still remains unknown. It is of crucial importance to widen the knowledge of physical processes in coastal regions to understand the ecology of larval echinoderms. Describing circulation and mixing processes through the water column along the Caribbean may contribute to understand larval dispersal.
The generalized low densities (< 1 Ind·m-2) after the mass die-off event along the Caribbean, may also influence the slow recovery reducing larval supply; taking into account that fertilization declines exponentially with decreasing densities (Allee effect) and that larger distance between individuals is a major impediment to gamete encounter in lower density populations.40 Further studies are needed to examine larval settlement, growth and parental source to better understand the low recruitment. The lack of or reduced recruitment of D. antillarumcan potentially threaten the recovery of the species and can leave themsusceptible to a potential localized extinction from a possible disease outbreak or severe weather event resulting from climate change.
Bathymetric segregation: In general, the Diadema antillarum densities presented higher values at shallower depths. Its abundance increased from the 0-4 m to the intermediate 4-6 m depth intervals and was almost absent for depths below 10 m. Although, some inconsistencies were detected in the vertical distribution patterns amongst localities, the results conclude a preferred depth range 1-7 m colonizing rugose rather than smooth substrate. Aggregations of this echinoid are found in less complex habitats, juveniles can be found inside the aggregates where they provide mutual protection between closely spaced urchins in open areas,10 aggregations in this study were observed at less exposed sites with moderate wave action and rocky substrate offering numerous caves and crevices to home these sea urchins at 0-3 m depth range, similar results were obtained by Rodriguez-Barreras et al.32 at Puerto Rico.
Bathymetric distribution for the different size ranges: The size-frequency distribution of Diadema in the “urchin zone” varied amongst the 4 localities as the habitat characteristics also differed in some parameters that may be influencing in the spatial distribution of D. antillarum populations. The D. antillarum size class 2 (pre-adults: 3-6 cm test diameter) was more evenly distributed along the “Diadema zones” (1-7 m).26,27 The juveniles (<3 cm) and adults (>6 cm) were more abundant at intermediate depths (4-6 m), increasing from the shallowest depth range (0-3 m) to a maximum abundance in the intermediate depth interval (4-6 m) and significantly decreased at depths from 7-10 m and deeper. Each community surveyed showed different patterns for the Diadema distribution, hence supporting the hypothesis that factors affecting their distribution appear to be more related to the turbulence of water, availability of shelter structures and suitable substratum and the number of predators or competitors for resources in the area (overfishing pressure).2,18
Sheltered areas, with moderate current and turbulence, low wave action, presented higher densities of D. antillarum. High wave energy and stronger current sites presented lower densities. D. antillarum was found more dispersed at more complex reef habitats and large numbers of individuals in aggregations were found at less complex, open habitats, where their conspecifics may provide protection. The pattern detected at Bayahibe, with lower densities of this echinoid at shallower depths (0-3 m) and increasing abundance with increasing depth up to 8 m, matched with the recent study by Soto & Irizarry at Puerto Rico, although the registered Diadema densities were higher (1.75-2.35 ind·m-2). Punta Cana D. antillarum abundance was lower compared to the other localities with an apparent recruitment failure, probably due to the strong currents characteristic from the Mona Passage and the strong wave action hindering larval settlement. Because the arrival of planktonic larvae at suitable settlement sites is primarily dependent on advective transport, both local hydrodynamics and large-scale oceanographic features are important determinants of recruitment success;37,41 the recommended continued monitoring of this specie should be linked to these abiotic factors on a widen spatial and temporal scale. Samana and Sosua with lower substrate heterogeneity may have limited suitable substratum availability. The results obtained at Sosua, at the island rock (SS1), with a 4 fold higher abundance of individuals of size class 2 could be related to a reduced volume of the individuals rather than the age; the overcrowded island could lead to a reduced size (TD) due to food limitation, which is consistent with Levitan42 and Garrido43 indicating that D. antillarum decreases its shell in situations of limited food.
Benthic cover: Localities surveyed presented similarities between Samana and Sosua, with predominant sediment coverage and low coral percentage values; the high sedimentation rates may impede coral recruitment and growth.44 All localities presented values close to a 20% macroalgal cover, no significant differences were found to relate with the echinoid abundance, indicating that Diadema population density seems to be independent from local resources.42
The greatest benthic cover complexity was observed at Bayahibe coinciding with a higher dispersed density of Diadema at this locality. Having into consideration that Bayahibe is a fishermen town, where sea urchin predator populations might be under fishing pressure; the rapid increase of this echinoid in Bayahibe could indicate the absence of main predators, 15 species of fish including ballistids, sparids, batrachoidids, Cassis helmet gastropod and spiny lobster Palinurus argus and other fish that strongly compete with Diadema for algal resources as some species of scarids and acanthurids.2,5,6,43
Furthermore, Gloeckler12 reported benthic cover values of 39.9% macroalgae and 11.1% coralline algae in Bayahibe, compared to the 24.69% and 8.40%, respectively, observed in the actual study. The decrease in photophilic macroalgal bed could be related to the Diadema increase, although an increase in crustose coralline algae would be expected.5,45 Bayahibe has a developed coastline full of Touristic Hotels and Resorts that grew without territorial planning; tourism overloads this location and consequently deteriorates water quality. Bayahibe subsoil consists mainly of extremely porous limestone, full of holes with dead coral and other fossilized organisms; drainage is excessive, making most of the rainfall quickly seep into deeper strata, with the poorly treated sewage possibly infiltrating the subsoil and ending in the marine environment. The latest Environmental Protection Program report (2014) denounced the organic pollution from the underground waters from Bayahibe.46 This location has also a great number of vessels taking hundreds oftourists daily to the Saona Island, which may be contributing also to impoverish water quality. Further research on how this may affect the reef communities is recommended.
Bayahibe was the only locality with comparable data of Benthic Cover, showing the dramatic loss in Coral communities, decreasing from a >30% in 2004-2006 to a 20%,34 15.3%12 and to 13.54% on the actual study; highlighting the urgent need for corrective measures to alleviate the processes that may be contributing to the demise of these essential communities. For this study a more accurate classification of the algal communities was not performed, but a surprisingly high abundance of cyanobacteria was found in both Bayahibe and Punta Cana. For statistical analysis cyanobacteria was classified as macroalgae. A more detailed and extended benthic cover study along the DR is recommended together with population densities of this echinoid to see if there are shifts in communities and their functional structure to better understand the multifactorial distribution of Diadema populations.
The author would like to thank the Puntacana Ecological Foundation for their logistical support, especially to Victor Galvan, Susanne Leib and Karolina Wikström for having contributed with their constructive criticism and comments and for helping in the field. The author would also like to thank the unconditional help of FUNDEMAR, especially to Rita Sellares and Alido Luis Baez. This work was financed by the Inter-American Development Bank (IDB) through the Multilateral Investment Fund non/refundable technical collaboration number ATN/ME:13126-DR, DR/M1035> Support for the Conservation of Coral Reefs through the Tourism Promotion of Coral Gardens. Additional support was provided by the United Nations Small Grants Program (Programa de Pequeños Subsidios, PPS in Spanish) grant number> DOM/SGP/OP5/COREBD/2012/18.
None.

©2015 Torres, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.