Journal of
eISSN: 2378-3184


Research Article Volume 12 Issue 2
1Laboratory of Ecology, Biology and Physiology of Aquatic organisms, Tunis Faculty of Science, University of Tunis El Manar, Tunisia
2University of Carthage, Higher Institute of Fisheries and Aquaculture of Bizerte (ISPAB), Tunisia
3Laboratory of Fisheries Sciences, National Institute of Marine Sciences and Technologies (INSTM), Tunisia
Correspondence: Feriel Ghribi, Laboratory of Ecology, Biology and Physiology of Aquatic organisms, Tunis Faculty of Science, University of Tunis El Manar- 2092 Tunis-Tunisia
Received: April 25, 2023 | Published: May 4, 2023
Citation: Ghribi F, Bejaoui S, Chetoui I, et al. Biometric relationships for the bivalve Ark shell Arca noae (Bivalvia; arcidae) from the Bizerte lagoon (Northern Tunisia). J Aquac Mar Biol. 2023;12(2):92-97. DOI: 10.15406/jamb.2023.12.00361
Bivalves are known to exhibit wide morphological variations, which are closely linked to their ecology and reproductive activity. Therefore, the study of growth is necessary to model population dynamics, which, in turn, is crucial to support the exploitation and management of the stock. The current study aimed to analyze the shell morphological variations in the bivalve Ark shell Arca noae collected monthly from the Bizerte lagoon (northern Tunisian coasts) from October 2013 to September 2014. Allometric relationships established between linear variables (shell length and height); weight variables (total weight) and the relative growth between variables (isometry vs allometry) were analyzed. All morphometric relationships were highly significant with high correlation coefficients (R2: 0.604-0.903). Seasonal monitoring of the linear and weight relationship of the A. noae population suggested the presence of a negative allometric growth during the seasons which indicated that Ark shells are growing faster in length than in height and weight. Overall, linear and weight growth in A. noae from the Bizerte lagoon was found to be uniform and synchronous over the seasons and seems not to be influenced by the species’ reproductive cycle. This preliminary study will be useful for further research studies in biology, ecology and stock management of this non-exploited marine resource.
Keywords: bizerte lagoon, Arca noae, shell morphology, relative growth
The knowledge of growth parameters is essential to understand the biology and productivity of bivalves. The study of growth is necessary to model population dynamics, which, in turn, is crucial to support the exploitation and management of stocks1 as well as the development of effective measures for the protection of species.2 The biometric study in relative growth aims to establish relationships in the form of mathematical equations between the different linear and weight parameters of a given species. Such studies provide numerous concepts about the relative growth of animals and the changes that can affect their development.3 Indeed, it is generally accepted that the morphological variables of individuals can reflect or correspond to certain biological variations. These morphological and biological variations are influenced, totally or partially, by environmental conditions, as has been demonstrated in several aquatic species.3–5
The bivalve Ark shell Arca noae has a great economic interest and is very appreciated by consumers on the northern shores of the Mediterranean. The Bizerte lagoon inhabits a wide variety of benthic species, including the bivalve Noah's Ark (Arca noae; Linnaeus 1758). Noah's Ark "Arca noae" belongs to the Arcidae family. It is an edible commercial bivalve characterized by an equivalent shell, strongly calcified, quite angular, without pallial sinus. A. noae is widespread throughout the Mediterranean Sea and in the Atlantic Ocean from Portugal to Angola.6 It lives attached by a solid byssus to solid substrates (rocks, shell debris) at a depth ranging from a few tens to more than 120 m.7 This species becomes sexually mature at an early age, 2 years, when it is slightly above 15 mm and 20 mm in males and females respectively.8 A. noae can reach a length of 12 cm and lives up to 25 years.5 It is the most important edible species of the Arcidae family in the Adriatic Sea and it reaches high prices in Croatia, Italy and Slovenia.9
Despite its importance, no studies have been carried out about the morphological variations in the A. noae population from the Bizerte lagoon. The present study aims mainly to determine the relative growth of the edible bivalve A. noae in the lagoon of Bizerte. This information is needed to establish a database for an unexploited (sub) population for stock management that may be exploited in the future. In addition, the information obtained from the study of unexploited stocks (example: A. noae from the Bizerte lagoon) gives an overview of the natural biological parameters of a species that constitute a reference tool that can be essential for the assessment and management of overexploited stocks in other geographical areas.
Sampling site
The Bizerte lagoon, known for its geostrategic position, is a semi-closed body of water that communicates with the Mediterranean Sea through a 6 km long channel and communicates with Lake Ichkeul (110 km2) through the Tinja channel, about 5 km long and a few meters deep (3 m).10 Our study area was selected primarily for easy access, the continued presence of A. noae with a relatively high abundance at a shallow depth (3 to 5 meters) as well as the distance of the prospected site from major sources of pollution. Our sampling site (coordinates: 37°08'36.0"N 9°52'20.4" E) is located in the southern part of the Bizerte lagoon, far from urban and industrial sources of pollution (Figure 1). This region has been assessed as the less contaminated area in the Bizerte lagoon but is still influenced by inputs from agricultural activities.11–13
Sampling procedure
A. noae specimens were collected monthly by scuba diving in the Bizerte lagoon (15th day of each month) using a specialized diver for a full year (October 2013-September 2014). Ark shells were immediately transported to the laboratory in a cooler. In the laboratory, specimens of A. noae were verified using a determination key designed for bivalves.9 Our specimens are sexually mature individuals (mature specimens have a shell length > 12 mm.14
Analysis of morphological parameters
To follow the evolution of the morphological characteristics of the animal's body during its annual biological cycle, we used the following measures:
The measurements considered were taken on each shell (Figure 2) using a calliper with an accuracy of 0.01 mm and by weighing using an electronic scale with an accuracy of 0.001 g.
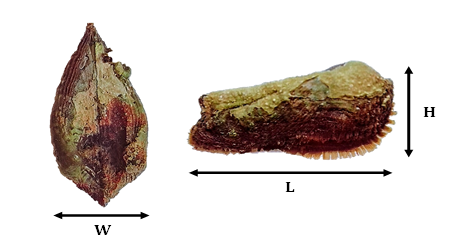
Figure 2 Biometric measurements of A. noae specimens from the Bizerte lagoon (L, length; H, height; W, width).
Analysis of morphometric relationships
In this part, we took into consideration all the samples collected during a whole sampling year (~ 791 individuals/ year). We tried to figure out if a morphometric variable influences another variable. More precisely, we studied if there is a relationship between each two chosen morphometric variables. This method of investigation on observational data, where the main objective is to find a linear link between a quantitative Y variable and one or more equally quantitative X variables; establishes a simple linear model after a logarithmic transformation. Therefore, we looked for possible relationships between linear and annual weight parameters.
Relative growth
All the measurements obtained allowed us to study the seasonal allometric relationships linking the different linear and weight variables. The seasonal allometric relationships used in this study concern length-height and length-weight relationships. The biometric method 15 makes it possible to establish the relationships that exist between the morphometric parameters and makes it possible to follow the growth of the animal over time.
Linear growth
After logarithmic transformation:
With: H, Height; L, Length
Weight growth
After logarithmic transformation:
With: TW, Total weight; L, Length
The parameters used in this relation, namely the y-intercept (a) and the slope (b), are evaluated by the method of least rectangles16 from the transformed data. To determine the nature of allometry, we compared the observed value of the slope (b) with the theoretical value 1 (value of the theoretical slope) (if it is about allometric relations linking two linear variables) or 3 (if it is a linear measurement and a weight measurement) using Student's t-test (at 5% as the error threshold).
Statistical analysis
The Student (t) test was used to check the different relationships relating to the linear and weight growth of the ark shells with the following formulas:
Linear growth
S2y, variance of y; S2x, variance of x; r, correlation coefficient; n-2, degree of freedom; Po, observed value of the slope; P, given value of the slope
Weight growth
b, the slope of the regression line; (b): the standard error of the slope
Two cases can arise concerning the t-test calculated for the two relations:
Analysis of morphometric parameters
In the current study, we measured several morphological parameters such as length (L), height (H), width (W) and total weight (TW). The values of these morphological parameters of ark shells collected during a whole year of sampling from the lagoon of Bizerte varied from 16.39 to 80.15 mm for the length; from 14.31 to 44.50 mm for the height; from 11.81 to 38.96 mm for the width and from 11.13 to 66.63 g for the total weight. The averages of the annual length (L), height (H), width (W) and total weight (TW) measurements of A. noae are summarized in Table 1.
|
Variables |
N |
Minimum |
Maximum |
Mean |
Standard error |
|
L (mm) |
791 |
16.39 |
80.15 |
56 |
0.31 |
|
H (mm) |
791 |
14.31 |
44.5 |
30.11 |
0.17 |
|
W (mm) |
791 |
11.81 |
38.96 |
27.41 |
0.15 |
|
TW (g) |
791 |
11.13 |
66.63 |
27.05 |
0.38 |
Table 1 Measurement of annual morphological parameters (L, H, W and TW) of Ark shells from the Bizerte lagoon
L, length; H, height; W, width; TW, total weight; N, number of individuals
Analysis of morphometric relationships
We found that the studied linear and weight variables can have relationships between them, revealing that points seem to follow a linear regression line (Figure 3). In A. noae, each morphometric variable directly influences the other variable (Figure 3). Multiple relationships were established between height and width; height and total weight; width and total weight; length and height; length and width and between length and total weight. All these morphometric relationships were highly significant with high correlation coefficients (R2: 0.852-0.911).
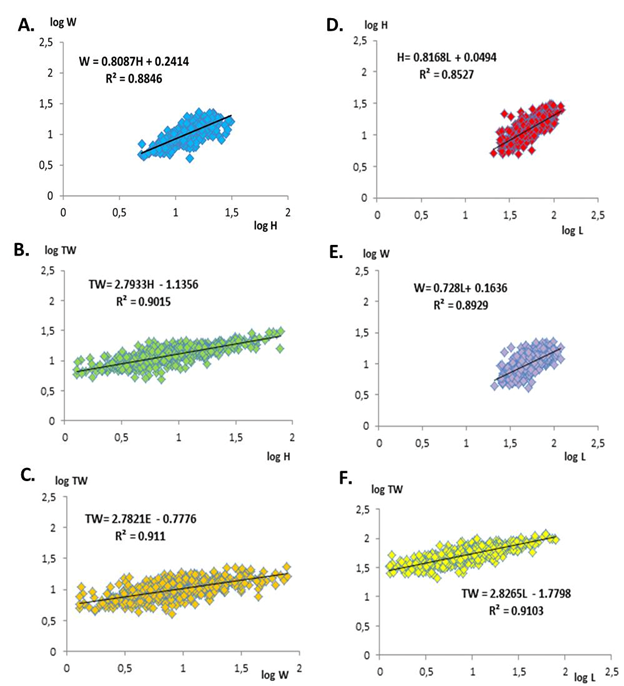
Figure 3 A. Biometric relationships: A. relationship between height and width; B. relationship between height and total weight; C. relationship between width and total weight; D. relationship between length and height; E. relationship between length and width; F. relationship between length and total weight.
Linear relative growth
The analysis of the linear variables (L/H) taken two by two, their ratio, as well as the type of allometry, are grouped in Table 2. The analysis of the relationship between the two morphological variables (Length-height) during seasons showed that the calculated value of t is higher than the theoretical value (1.96). The slope (b) of the regression curve is less than 1, which reveals a negative allometric relationship between length and height during the seasons (Table 2). The growth of the length of the shell during the seasons evolves at a higher rate than that of the height. The values of the correlation coefficient (r) which link the different linear variables were between 0.604 (p <0.05) and 0.903 (p <0.05), which testified to a significant correlation between length and height. While the L/H ratio varied between 0.52 and 0.54.
|
Season |
N |
Relation |
Equation |
r |
t |
Type |
Ratio |
|
Autumn |
90 |
L/H |
H =0.631L0.960 |
0.903 |
2.012 |
negative allometry |
0.52 |
|
Winter |
90 |
L/H |
H =0.653L0.866 |
0.801 |
2.846 |
negative allometry |
0.54 |
|
Spring |
90 |
L/H |
H =0.538L0.817 |
0.818 |
3.873 |
negative allometry |
0.54 |
|
Summer |
90 |
L/H |
H =0.770L0.558 |
0.604 |
6.636 |
negative allometry |
0.53 |
Table 2 Seasonal variation of the linear relationship between the length and the height of the Ark shells collected in the lagoon of Bizerte
N, number of individuals per season; L, shell length; H, height of the shell; t, student test significant at the 5% level; r, correlation coefficient
Relative weight growth
The study of weight growth is based on the analysis of the relationship between the two parameters total weight and length. The correlation coefficient values are between 0.685 and 0.858, showing a significant relationship between these two parameters (Table 3). The calculated t value was higher than the theoretical value (1.96), which reveals an allometry between length and total weight. The negative allometric growth (b <3) observed between the total weight and the length indicates that the length of the shell increases faster than the total weight of the animal during the seasons.
|
Season |
N |
Relation |
Equation |
r |
t |
Type |
Ratio |
|
Autumn |
90 |
L/TW |
TW=0.338L2.538 |
0.858 |
21.681 |
negative allometry |
0.41 |
|
Winter |
90 |
L/TW |
TW=0.353L2.260 |
0.799 |
32.897 |
negative allometry |
0.50 |
|
Spring |
90 |
L/TW |
TW=0.335L2.258 |
0.757 |
31.313 |
negative allometry |
0.51 |
|
Summer |
90 |
L/TW |
TW=0.344L1.990 |
0.685 |
35.783 |
negative allometry |
0.43 |
Table 3 Seasonal variation of the weight relationship between the length and the total weight of the Ark shells collected in the lagoon of Bizerte
N, number of individuals per season; L, shell length; TW, total weight; t, student test significant at the 5% level; r, correlation coefficient
Seasonal monitoring of the linear relationship in A. noae (L/H) from the Bizerte lagoon suggested the presence of hypoallometric growth during the seasons. Also, the study of the weight relationship (L/TW) showed the existence of hypoallometric growth between the length and the total weight of the animal during the annual year of study. The negative allometry (L/H relationship) showed that it is growing faster in length than in height. This explains the elongated shape of the shell along the anteroposterior axis. Similarly, the negative observed allometry between the length and the total weight of ark shells showed that the length of the shell increases faster than the weight of the animal. The values of the correlation coefficient (r) which link the different linear and weight variables show a good correlation between the length/height and the total length/weight. Indeed, the values of the correlation coefficient (r) obtained for all the studied parameters (TW, L and H) varied between 0.604 and 0.903. These values revealed the close relationships existing between the three linear and weight parameters.
Our results are in agreement with the work of Dridi et al.17 and Derbali et al.4 on the bivalves Crassostrea gigas and Pinctada radiata from the Bizerte lagoon and the Kerkennah islands. Indeed, a linear growth (Length/Height) with negative allometry type was observed in P. radiata collected from the Kerkennah Islands.4 Dridi et al.17 showed that the population of C. gigas in the Bizerte lagoon is characterized by faster growth in length than in height (negative allometry). The same type of growth was also observed by other authors who studied the growth of clams Ruditapes decussatus in Spain18 and mussels Mytilus galloprovancialis in the Bizerte lagoon.19 Nevertheless, our results are in disagreement with the work of Gaspar et al.20 where a linear growth of positive allometry or isometry type was observed in Acanthocardia aculeata, Acanthocardia tuberculata, Modiolus adriaticus and Venerupis rhomboides from the Portuguese coasts. However, our results on weight growth (negative allometry) in A. noae disagree with the work of Chetoui21 who showed that the weight growth of the bivalve Mactra corallina collected from Kalâat El Andalous followed the isometric growth. Mzighani22 showed that Ark shell Anadara antiquata from Tanzania grows at the same rate in all linear dimensions (isometric growth). This same author also reported that a correlation coefficient r; which is very close to 1, indicates a high degree of correlation between the morphometric variables analyzed. Not only length and weight are important, but other morphometric parameters (such as shell height and width) are also important factors in comparing the growth of a population which can also be used to monitor changes within a given species. In general, some disagreements on the morphometric relationships between species may be the consequence of distinct hydrological and sedimentological characteristics between different geographical areas. Additionally, the size ranges of some bivalve species are highly variable across several studies, further complicating comparisons between species from different geographic regions. These considerations confirm that morphometric relationships are highly variable between bivalve species and between different geographical areas.20
In this study, linear and weight growth of A. noae from the Bizerte lagoon was found to be uniform and synchronous over the seasons with greater growth in length than in height and in total weight. The values of the correlation coefficient (r) which characterize linear and weight growth remained high and constant during the annual reproductive cycle of this species. According to our results, the linear and weight growth of the ark shells seems not to be influenced by the reproductive cycle. On the other hand, in the work carried out by Dridi et al.17 on C. gigas from the Bizerte lagoon, the values of r varied significantly. Low r values were observed during reproduction and high values during sexual rest, indicating better linear and weight growth outside the sexual activity of the gonads. During sexual rest, C. gigas seems to spend a large part of its energy on growth, while during sexual activity this energy would be rather solicited for the process of gametogenesis from which growth is better outside the reproductive phase.17 It is generally accepted that growth is characterized by an increase in height and weight over time. However, several other factors influence linear and weight growth in bivalves such as the physical and nutritional state of the environment,23 the physiological state24 and the genetic parameters of the species.25
The biometric method has been used by several authors to study the morphometric diversity of a species in several localities. The difference in morphometric characters between populations from different sites has been widely reported in previous studies. Generally, population divergence has been explained by the physiological properties acquired by a given species to adapt well to the environmental conditions of each habitat.4 Belkhodja and Romdhane26 analyzed the morphometric diversity of the gastropod mollusc Patella caerulea inhabiting three rocky sites on the northern coasts of Tunisia (Bizerte Canal, La Goulette and Sidi Rais). This study showed that overall, there is a significant difference between the morphometric characteristics of the three populations studied and which is probably due to an adaptive response to the physical and ecological conditions of the environment. Several biotic and abiotic factors influence the morphometry of bivalve species. In the present study, the use of this morphological study has provided new information on the evolution of morphometric parameters in A. noae from the Bizerte lagoon. The study of the intraspecific polymorphism of biometric characters in this bivalve is envisaged in subsequent works.
Several studies have been conducted on the absolute growth (growth rate and model by counting the ridges, age estimation, etc.) of populations of A. noae in the Adriatic Sea (Croatia)5,27,28 but never on the relative growth of this species. Several methods have been used to determine absolute growth in populations of A. noae in the Adriatic Sea27 based on the reading of the outer surface growth rings, the study of the pallial line scars on the inner surface of the shell and the lines and bands of growth in acetate replicas of the umbo region and the outer layer of the shell. Indeed, the work carried out on the growth rate and age of A. noae in the Adriatic Sea (Croatia) showed that Noah's Ark is a slow-growing species reaching a maximum size of 70 mm and an age of up to 16 years in sites where fishing is frequent and whose populations are highly vulnerable due to overexploitation.27 Whereas, in a marine protected area in Telaščica Bay in Croatia, ark shells can reach a length of 120.3 mm and be 25 years old.5 The sighting of large specimens in this region has been linked to the lack of fishing efforts. According to Peharda et al.,27,28 the lifestyle adopted by A. noae influences its age and growth rate. Indeed, variations in the growth rate of A. noae relative to different locations (Malo Jezero, Marina and Mali Ston Bay in the Adriatic Sea, Croatian coasts) have been observed.27 A. noae collected at “Malo Jezero” has low growth and reaches a smaller maximum size compared to specimens from “Marina” and “Mali Ston Bay”. This same author suggests that the low growth rate observed for the population of "Malo Jezero" can be attributed to competition for resources associated with a group lifestyle where individuals are attached (cluster model). While at “Marina” and “Mali Ston Bay” A. noae were more commonly solitary attached to hard rocky substrates, reflecting a lifestyle marked by less competition between individuals. Broom29 studied the growth of several species belonging to the genus Arca and concluded that growth in these sedentary and semi-sedentary bivalves is strongly influenced by environmental and biological factors and varies considerably from population to population, between individuals; even within the same species and the same population. The slow growth rate of abundant molluscs and crustaceans is probably related to competition for food and living space.30,31 Differences in growth rate between populations may reflect acclimation to ambient habitat conditions or genetically established adaptation within populations of the same species. Indeed, in a given geographical site, each population acquires physiological properties for adaptation to environmental conditions such as the abundance of food, temperature, salinity and pollution.4 Further studies looking at the absolute growth and eco-biological traits of A. noae in the Bizerte lagoon (abundance, density, population structure, way of life etc.) are strongly recommended.
The present work was devoted to studying the relative growth of the non-exploited population of A. noae in the Bizerte lagoon. The high values of the correlation coefficient (r) obtained at the level of weight and linear growth highlighted the strong relationship existing between the different variables. Seasonal monitoring of the linear and weight relationship of Ark shells suggested the presence of a negative allometry among seasons. This suggests that this bivalve is growing faster in length than in width and weight. In general, linear and weight growth of A. noae from the Bizerte lagoon was found to be uniform and synchronous over the seasons and seems not to be influenced by the species’ reproductive cycle. Further analyses are required to determine the dynamics structure and age characteristics of A. noae populations. To conclude, it will be interesting to investigate other populations from Tunisian coasts for better data collection and Ark shell stock evaluation. This preliminary study will be useful to maintain reasonable stock exploitation and management of this species.
Dr Bejaoui and Dr Chetoui contributed equally to this work.
None.
The authors declare no conflicts of interest.

©2023 Ghribi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.