Journal of
eISSN: 2378-3184


Case Report Volume 11 Issue 3
1Laboratory of Marine Geology and Geophysics and Environmental Monitoring (GGEMMA), Federal University of Rio Grande do Norte (UFRN), Brazil
2EcoLogicProject.com, USA
3Graduate Program in Environmental Science and Technology (UNIVALI), Brazil
4Environment and Geomatics Lab (IFSC), Brazil
5Dynamic Bilingual School, R Alves de Brito, Brazil
6Oceanographic Center for Stratigraphic Records of the São Paulo Oceanographic Institute (IOUSP) São Paulo-SP, Brazil
Correspondence: Patricia Pinheiro Beck Eichler, Laboratory of Marine Geology and Geophysics and Environmental Monitoring (GGEMMA), Federal University of Rio Grande do Norte (UFRN), University Campus, PO Box 1596; CEP 59072-970, Natal - RN, Brazil,
Received: November 04, 2022 | Published: November 16, 2022
Citation: Eichler PPB, Corrêa AA, Barker CP, et al. Analysis of benthic foraminifera in the reproductive area of right whales (Eubalaena australis) in the Western South Atlantic. J Aquac Mar Biol. 2022;11(3):113-123. DOI: 10.15406/jamb.2022.11.00345
Susceptibility and vulnerability to impacts make Cetaceans and Benthic Foraminifera sentinels of health in marine environments. A breeding population of Right whales (Eubalaena australis) annually migrates to the “Environmental Protection Area” in Santa Catarina, Brazil, where Ribanceira/Ibiraquera Bay, and its harbor, are located. Since Right whales rest in shallow areas, their stomachs touch the marine sediment bottom, where foraminiferal assemblages inhabits. Temperature, salinity, pH, turbidity, grain size, morphometry, and magnetic susceptibility correlated to foraminiferal species, contribute to the understanding of habitat preference for right whales. Here we show saline waters in the northern areas associated with Pseudononion atlanticum, Elphidium sp., Buccella peruviana, Quinqueloculina patagonica, and 21°C of temperature is the preferred by mother and calf pairs. The harbor has lower pH, higher temperatures, magnetic susceptibility, and turbidity, and high depth is due to dredging. These characteristics do not support the presence of the Right whales, the top of the food pyramid, so decline or increase in their population indicates changes in their habitat. We stress the importance of unravelling signals of Benthic and Nekton coupling by understanding whales’ habitat; to ensure the recovery of their populations, and the survival of other species in the marine ecosystem.
Cetaceans are considered excellent indicators of health in marine ecosystems1 and are susceptible to various threats of anthropic origin such as habitat degradation, pollution of aquatic environments, increased vessel traffic and interactions with fishing nets.2
A reproductive population of right whales (Eubalaena australis) frequents the southern coast of Brazil every year during the winter and spring months and shows preference for some inlets on the coast of Santa Catarina to perform mating and parental care activities.3–6 These mammals move slowly between inlets where they are often visible on the surface, and remain in the region for a few weeks and/or months.7,8 Due to the behavior of close to coastal proximity, especially the female and calf pairs, these animals are extremely vulnerable to coastal human activities. The loss of habitats of reproductive importance represents one of the greatest threats to cetacean species.9,10
Areas frequented by right whales, such as these reproductive concentration areas of southern Brazil, require special protection due to the vital importance of carrying out their reproductive cycle, while also aiming at population recovery and reoccupation of their distribution areas.2,11 Because they live and thrive within a conservation area for sustainable use, that is, a space shared between society and cetaceans; there are interactions through water sports, port activities, fishing and tourism. It is essential to study and monitor the environmental quality of the species, as they are an active element for the sustainable development of the region. Studies on the influence of environmental factors on the distribution of right whales in reproductive areas have described some common chemical and physical factors of the environment in relation to habitat use. For southern right whales, factors such as depth and slope,12 calm waters and absence of rocks,13,14 protection from wind and waves, sandy bottom and shallow depth,15 prove importance in studies of habitat use. Recent studies of humpback whales preference in the reproductive area of Hawaii, for example, have also found a relationship between depth and type of seabed.16,17
Some whales, such as the Gray whales, do not feed on the ocean floor.18 They roll to one side and swim along the bottom, then aspire (by pressing the tongue) the sediment and prey where sediment and water are filtered out by the whale, indicating this benthic feeding behavior.19 This disturbance of the sediment by feeding whales changes the composition of sediments and supports the idea that this behavior shapes the structure of the substrate and sand and helps maintain balanced levels of the amphipods, their main source of prey in some areas of study.20 Gray whales scour the seabed when they feed and this process leads to the resuspension of sediments and nutrients that would otherwise remain on the seabed. Therefore, although this feeding may seem like a violent disturbance, it may in fact play a big role in benthic productivity.20,21
Due to the inherent interrelationships between geological, physical, chemical and biological in marine environments, the use of benthic organisms as indicators is regarded as a good alternative for environmental studies. These indicator organisms, the benthic foraminifera, are single-celled, capable of synthesizing the general characteristics of the environment, highlighting the environmental variations of short periods and reacting to seasonal variations and anthropic effects,22 thus allowing the realization of environmental analyzes that make it possible to recognize local problems. In these coastal environments, biological communities are structured mainly by physical-chemical conditions, such as: wave energy, tidal regime, slope, sediment sizes, temperature, salinity, and dissolved oxygen, so that natural or anthropogenic changes can significantly affect such communities. This makes it essential for constant monitoring of these variables so that the quality of the environment may be understood and evaluated. Also, in addition to monitoring whales, foraminifera as bio indicators is an essential way in which the quality of the environment can be measured. Unlike whales, foraminifera have a short life cycle and a rapid response to environmental variations, their distribution is mainly controlled by physical-chemical variables. From characterizing the fossil foraminifera, which indicates environmental quality (average conditions of the last year) the preferential inlets of right whales is shown to highlight aspects of the Benthic Nekton Coupling (BNC) that exists in the region. This mechanism of BNC is translated by the set of exchange processes existing between the nektonic and benthic systems in the marine environment, which is influenced by seasonality, and the pattern of nutrients between the pelagic photic zone and the marine sedimentary column.23 Therefore, the processes that occur in marine sediment can influence environments selected by right whales in the reproductive period, and we intend to evaluate them.
Here we want to describe aspects of sediment and water in two coves where right whales inhabit, using the analysis of foraminifera, sediment sizes, morphometry, and magnetic susceptibility of the sediment, in addition to the physical-chemical, and hydrographic parameters (temperature and surface salinity, pH and turbidity). We will also discuss which natural geomorphological, hydrographic and anthropic aspects might be contributing to studies on the use of this species' reproductive habitat areas in southern Brazil.
The research was carried out in Imbituba, south-central coast of Santa Catarina, central area of the Baleia Franca Environmental protection area (EPA) (Figure 1). The Ribanceira/Ibiraquera bay (28º11´34,31´´S - 48º39´28,71´´W) or (28 ° 10'S - 48 ° 39'W) is located to the north of Harbor beach, limited by rocky mountains with an extension of approximately 5.15 km. The northern portion of the cove is called Ibiraquera, where the Batuta Island and the Ibiraquera Lagoon bar are located. It has an extensive range of dunes and urbanization occurs further from the beach line. The southern part of the cove is called Ribanceira, where the Ponta da Careca do Velho islet (Creca) is located, and has an extensive string of post-beach dunes where urbanization is closer to the beach line. The Ribanceira / Ibiraquera cove has one of the highest concentrations of mainly pairs of female and young right whale groups in Brazil.24 Some recent works discuss aspects that may contribute to this preference The Porto beach (28º13´20,50´´S - 48º39´63,51´´W) is located south of Ribanceira/ Ibiraquera and has approximately 2.2 km extension. In this portion of the cove the Port of Imbituba is located, where there are 3 rocky jetties perpendicular to the beach and another jetty that serves as a breakwater, which extends for about 1 km from the end of the rocky shore parallel to the beach. Urbanization on this stretch is very close to the beach line. In the northern part of the cove, urbanization is farther from the beach line and ravines and gullies predominates on a high slope. The slope of the beach face, in the two coves, varies between 3 and 5 degrees,25 and the beaches can be classified as dissipative.26 The distance between the coastline and the 20 m isobath is approximately 5.5 km, based on data from nautical chart n ° 191001. In the northern portion of the coves the distance between the coastline and the 20 m isobath is approximately 3 km, showing an increase in the declivity of the beach face.
Off the Ribanceira/Ibiraquera bay, the Brazilian surface current flows over the shelf or close to the region where the shelf breaks and Tropical Water (AT) resides. This current flows southward along the east coast of South America, reaching an average of 38 S latitude, which is the Falkland Islands current, known as Sub Antarctic Shelf Water (SASW); in this region, which varies according to the time of year, the two currents move away from the coast, flowing in an easterly direction.
On the continental shelf located close to this coast27 describe three water masses which play an important role in this area dynamic. South Atlantic Central Water (SACW), the warm Tropical Water/Subtropical Shelf Water (STSW)/Brazil current flowing southward and the cold (Sub Antarctic Shelf Water (SASW)/Malvinas Current) flowing northward under the Brazil Current, together with Coastal Water (CW) and fresh water from Plata River (Argentina). This combination of water masses forms a perfect habitat providing means for the ecosystem to sustain life and biodiversity.
Sampling: On January 23, 2018 on board a twin-engine launch, 25 sediment samples were collected and environmental parameters (depth, temperature and surface salinity and sediment granulometry) were measured at 10 points in Ribanceira, 10 points in Ibiraquera and 5 points at the Port of Imbituba (Figure 1). The stations were positioned with GPS in the field, on board a small vessel, taking into account the depths of 7 and 14 meters, with the aid of an echo sounder. In addition, 5 water samples were collected for pH and turbidity parameters. Sampling for the analysis of parameters in the sediment and for the microfauna of foraminifera was performed with a Van Veen bottom catcher with a 50 cm3 sample area.
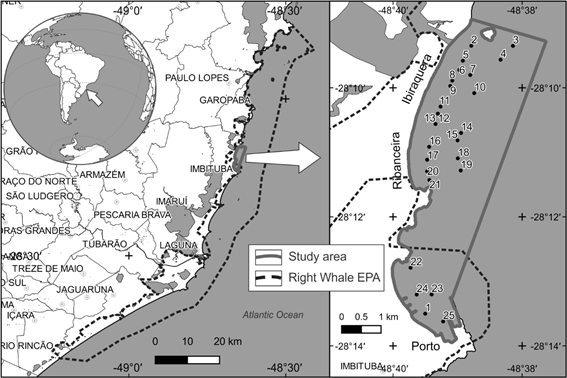
Figure 1 Location of Ribanceira/Ibiraquera and Porto bays in Imbituba-SC, highlighting the boundaries of the Right Whale EPA and the study area.
Processing of biological samples: The top layer (first centimeters) of each sample was removed and placed in bottles with Bengal Rose (1g / 1000ml of alcohol) for analysis of foraminifera microfauna. In the laboratory, the samples were sieved and washed using two successive 0.500 and 0.062 mm sieves and kiln dried. After separation, foraminifera were transferred with a brush to special black background slides (screening), for further analysis and identification of species. Species were determined using a binocular loupe on a 0.062mm sieve, referring to available taxonomic books.
Granulometry, morphometry and magnetic susceptibility: The equipment used to perform granulometry and morphometry analyzes is the Bluewave SIA- Dynamic Image Analyzer, Microtrac coupled equipment . Bluewave is responsible for particle size and measures particles using laser diffraction. The SIA characterizes the shape of the particles in liquid suspension through the analysis made by the PARTAN software of the frames of the filming performed. For the sediment refractive index, 1.54 (quartz, predominant in the area) was used and the liquid in which the particles were in suspension was water, so the refractive index of 1.33 was used. For the analysis of the particle size distribution and related statistics, the GRADISTAT version 4.0 program, developed by Blott, 2001 was used. The results shown here were calculated according to Folk and Ward.28,29
The low-field magnetic susceptibility normalized by volume () was measured using three frequencies of the Kappabridge MFK1-FA (AGICO).
The sediment samples were analyzed in partnership with the team from the Oceanographic Center for Stratigraphic Records (CORE) of the Oceanographic Institute of the University of São Paulo (IO-USP).
Numerical analysis: The descriptive statistical analysis of the microfauna was performed by calculating absolute and relative frequencies. Distribution maps were prepared for sedimentological data (granulometry and morphometry), biological data (relative frequency of indicator species), chemical data (pH) and hydrographic data (salinity and temperature), from the import of data into the QGIS 3.2 software. along with the nautical chart and converted to the shapefile format. Then, weighting interpolation of the inverse of distance (IDW) was carried out, distributed according to the minimum and maximum values of each variable and species and the isolines were extracted for cartographic production. Univariate and multivariate methods were applied to biological, sedimentological, physical and chemical data, calculated by the PRIMER 6 program30 to assess the community structure and classify the environmental variables that most influence it. Among the univariate methods are the total number of species (S), organisms (N), Shannon-Wiener diversity index (H '), Simpson dominance and Pielou Eveness (J), based on the absolute frequency of foraminifera species. Among the multivariate methods are the principal component (PCA) and multidimensional (MDS) analyzes, applied to abiotic data and the cluster analysis, applied to biological data. BEST analysis correlated environmental variables with biological data to determine the variable that most influences foraminifera fauna in this region. The data of the environmental variables were normalized log (x + 1) and ordered in the PCA. In the first PCA, all samples were considered in the analysis, and in the second PCA, stations 23 and 25 were excluded from the analysis, as they have a different pattern from the general one. Data on absolute species abundance were calculated for foraminifera samples and submitted to Cluster analysis to define foraminifera associations. The Bray-Curtis distance was used to measure the proximity between the samples and Ward's method was used to arrange the samples within a hierarchical dendrogram.
Abiotic Data: The collected samples were distributed in 2 coves in the central area of the APA da Baleia Franca - SC, with Ribanceira / Ibiraquera being a preferred area for right whales and the Porto cove an area with constant traffic by the maritime fleet, due to the Port of Imbituba, with fewer right whales. During sample collection (Table 1), at depths ranging from 5.5 to 17.1 meters, the recorded water surface temperature varied between 19.58 and 21.35 degrees Celsius at the 25 sample points and the salinity between 34 and 39 UPS. Regarding water samples (n = 5), the predominant pH was 7.8 and a point within the port area showed pH 7.67 (# 25). Turbidity, on the other hand, ranged from 22 to 29, with the lowest value also in the port area (# 25).

Table 1 Location data, latitude, longitude, depth, temperature, salinity, pH, turbidity, grain size (MV, MN, MA), sphericity, L/W Ratio, Convexity, Concavity, Circularity, roundness, and magnetic susceptibility (KRe [SI]) of sampling stations
The granulometric curve of all samples is symmetrical and platicurtic. The samples are polymodal and very poorly selected, with a dominance of clay-sized particles, with the exception of # 17, which has a predominance of very fine silt. The size Medi the range from 8.82 (# 17) and 11:51 φ (# 23), with a standard deviation ranging from 2.97 (# 23) 4:59 φ (# 17). The percentage of sand-sized grains ranges from 0% (# 23) to 17.6% (# 17). Coarse sand is found in only one of the samples (# 17). Regarding the morphometric variables, the sphericity of the grains showed little variation (0.84 (# 17) to 0.87), as well as rounding (0.65 (# 17) to 0.70). In addition, the magnetic susceptibility recorded in the study area ranged from 1.00 to 7.33 SI, where we verified a gradient of decreasing values towards the north of the area. Higher values were found at Porto beach (Table 1).
In the first principal component analysis (PCA-A) (Figure 2A), the abiotic variables appeared to be responsible for 63.4% of the variability of the seasons and samples 23 and 25 showed a pattern quite different from the others, with exceptions, with depths higher salinity, lower mean particle size and magnetic susceptibility, lower sphericity and roundness, and were excluded. Station 6, despite also having lower values of magnetic susceptibility, sphericity and roundness, has low depth and low salinity and was not removed for the second analysis.
The second principal component analysis (PCA-B) (Figure 2B) revealed that, according to Table 2, the cumulative variation of the environmental variables of PC1 and PC2 explains 63.5% of the variability of the samples (Table 2). Figure 2B is the graphical representation of table 3 that shows positive and negative correlations of the variables and the analyzed stations. Depth, temperature, magnetic susceptibility (KRe [SI]) seem to directly affect seasons 4, 6, 5, 10, 7, 3, 19. The salinity and grain size seem to be the abiotic factors influencing seasons 1, 14 , 13, 16, 20, 13, 17. Sphericity and rounding seem to directly affect stations 11, 12, 8, 2, 9. On the other hand, stations 15, 21, 18, 22, 24 seem not to be influenced directly by abiotic variables (cumulative variation of PC1 and PC2 explains 63.5% of the variability of the samples).
PC |
Eigenvalues |
%Variation |
%Variação cumulativa |
1 |
5.76 |
44.3 |
44.3 |
2 |
2.48 |
19.1 |
63.4 |
3 |
1.51 |
11.6 |
75 |
4 |
0.853 |
6.6 |
81.5 |
5 |
0.717 |
5.5 |
87 |
Table 2 Data from the analysis of the main component (PCA B), in which the cumulative variation of PC1 and PC2 explains that 63.5% of the variability of the samples is influenced by environmental variables
Variables |
PC1 |
PC2 |
PC3 |
PC4 |
PC5 |
Depth (Prof) (m) |
-0,231 |
0,244 |
0,267 |
-0,296 |
0,618 |
Temp. Sup. |
-0,132 |
0,268 |
-0,427 |
0,199 |
0,023 |
Sal. Sup. |
0,012 |
-0,486 |
-0,133 |
-0,021 |
0,565 |
MV |
0,296 |
-0,182 |
0,108 |
-0,539 |
-0,064 |
MN |
-0,307 |
0,234 |
-0,101 |
-0,397 |
-0,103 |
MA |
0,353 |
-0,194 |
0,138 |
-0,310 |
0,036 |
Sphericity |
-0,324 |
-0,340 |
-0,072 |
-0,090 |
0,034 |
L/W Ratio |
0,236 |
-0,082 |
-0,469 |
-0,354 |
-0,295 |
Roundess |
-0,280 |
-0,296 |
-0,390 |
-0,108 |
0,010 |
Convexity |
-0,347 |
-0,219 |
0,225 |
-0,083 |
-0,263 |
Concavity |
-0,286 |
-0,095 |
0,464 |
-0,082 |
-0,344 |
Circularity |
-0,359 |
-0,292 |
-0,126 |
0,047 |
-0,056 |
KRe[SI] |
-0,229 |
0,392 |
-0,171 |
-0,410 |
0,053 |
Table 3 PC data and environmental variables obtained in the analysis of the main component (PCA B)
Relative frequency: Fifteen species of foraminifera were recorded in the sampling carried out in the study area, with 9 stations (2, 5, 6, 9, 11, 12, 13, 20 and 24) being sterile. The species peruviana Buccella , Elphidium sp. , Pseudononion atlanticum and Quinqueloculina spp. were the predominant foraminifera species in the sampled seasons, followed by Bolivina spp. , Miliamina fusca , Miliolinella subrotunda , Reophax and Textularia earlandi (Table 4).
Representative maps: Figure 3 (A–C) shows that pH and turbidity present a gradient with an increase in the values of these variables from south to north. On the other hand, magnetic susceptibility shows a decrease in these values towards the north (Figure 3D). Stations 11, 12, 13 have a higher pH (Figure 3A). Figure 3B shows the delimitation of the area of maximum turbidity where a region similar to the estuary is located in season 5 and also a gradient that relates to the supply of fresh water from the Ibiraquera Lagoon bar. It is observed front between two different bodies of water. Station 17, on the other hand, has a higher particle size average (standard deviation). Magnetic susceptibility values are higher near the port, with pH and turbidity being lower.
The distributions of Textularia earlandi, Triloculina trigonula, Clavulina sp., Quinqueloculina lamarckiana, Rephax sp., and Bolivina striatula show similarities and are found in a region of medium turbidity (Figure 4A). Station 16 shows an increase in Textularia earlandi, Triloculina trigonula, and Clavulina sp. (Figure 4A–C). The species T. earlandi and Clavulina sp are non-selective and agglutinate grains of different sizes in their shell, being characteristic of lower salinity environments. Triloculina trigonula, a calcareous species with perforated wall, also seems to tolerate this shelf-like estuarine environment. Quinqueloculin lamarckiana, Rephax sp., and Bolivina striatula show an increase in occurrence in station 17, where the average, standard deviation, and percentage of sand are higher.
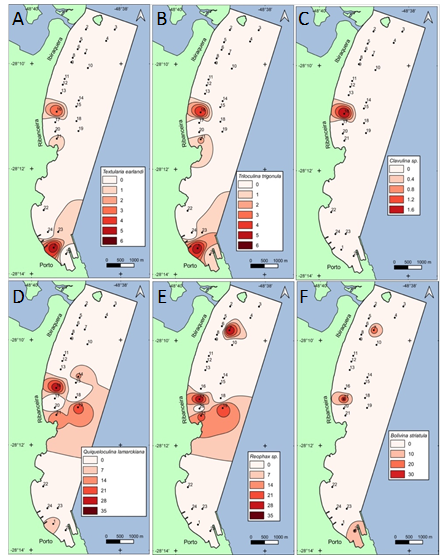
Figure 4 Data from (A) Textularia earlandi, (B) Triloculin trigonula, (C) Clavulina sp, (D) Quinqueloculin lamarckiana, (E) Rephax sp, (F) Bolivina striatula.
Medium turbidity area presents fresh water and greater diversity of species, as we will see in Figure 5. Figures 5A and B show that diversity and evenness are highest in the medium turbidity area (Figure 3B). In the southern part, we observed an increase in diversity where we observed the appearance of Miliolinella subrotunda (Figure 5C) and Miliammina fusca (Figure 5D). There is an increase in Miliamina fusca in a region of high diversity and medium turbidity. Figure 5 (E–G) shows lower salinity and roundness, respectively, in a region of medium turbidity. We observed that northern and southernmost parts are more saline than central part of the beach and that station 22 is one of the stations with higher salinities (Figure 5E, F). In addition, we observed that near station 24, where domestic sewage is being discharged, there is low diversity and evenness. It is observed that our samplings were performed between 6 and 15m depth isobates (5H).
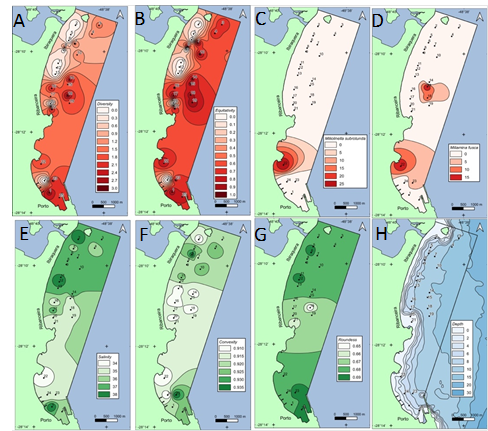
Figure 5 Data on (A) diversity, (B) equitability, (C) Miliolinella subrotunda, (D) Miliammina fusca, (E) salinity, (F) convexity, (G) roundness, (H) bathymetry at the collection stations.
Figure 6 shows that distribution of Pseudononion atlanticum (A), Elphidium sp. (B), Buccella peruviana (D), and Quinqueloculina patagonica (E) is highest where temperature is around 21°C (C). Northern part has colder temperature than the southern part and Elphidium sp. has higher numbers where temperature is less than 21°C (Figure 6B). Station 17 has the highest mean value (Figure 6F) and highest standard deviation values (seen in Figure 3C). In Figure 6 (G–I) we observed high dominance (I) in the northern part of the study. In the southern part (Figure 6F, G–I), close to the harbor, there is two types of sediment with different patterns: stations 24 and 1 with higher mean values in the Harbor and slightly higher dominance, and stations 23 and 25, which have lower average value and little or no presence of sand. We also observed decrease and absence of Buccella peruviana (D) and Quinqueloculina patagonica (E) where the average values are higher in the Harbor. Near station 24, where domestic sewage is released, there is high dominance of organisms. We also observed Buccella peruviana (D) and Quinqueloculina patagonica (E) being competing species. One of them flourishes in the decrease and absence of the other.
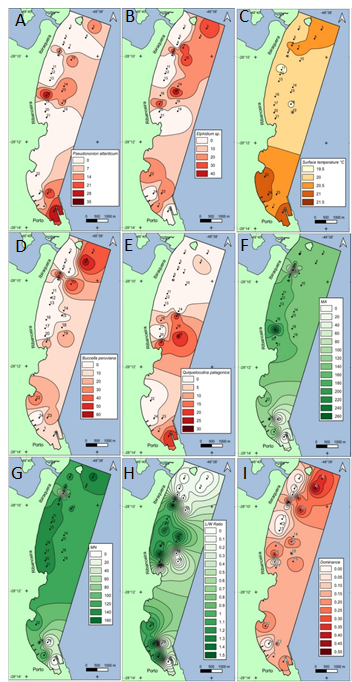
Figure 6 Distribution of Pseudononion atlanticum (A), Elphidium sp. (B), Temperature (C), Buccella peruviana (D), Patagonian Quinqueloculin (E), M / A (F), MN (G), L / W (H), and dominance (I).
Figure 7 shows Bulimina marginata (A) and Nonion sp. (B) occurring in the central part of the study region. This region shows transition region between three water masses: cooler and saline water in the north, fresher and warmer water in the south and saline water with medium temperature in the central part. These foraminiferal species are tolerant to this transition region of fresh and salt water, warm and cold waters. Distribution of Bolivina ordinaria (7C) in the same location as Bulimina marginata (7A), and Nonion sp. (7B), occurs also in station 16, are exact same site where Textularia earlandi, Triloculina trigonula and Clavulina sp. (Figures 6A–C) dominates, occurring also in the northern part the coldest region (station 3). We observed that in figure 7, sphericity values (7F) are lower in places where there is greater amount of fresh water, central part and stations 22 and 2, in the region of domestic sewage (Table 5).

Figure 7 Distribution of Bulimina marginata (A), Nonion sp. (B) Ordinary bolivina (C), concavity (D), circularity (E), and sphericity (F).
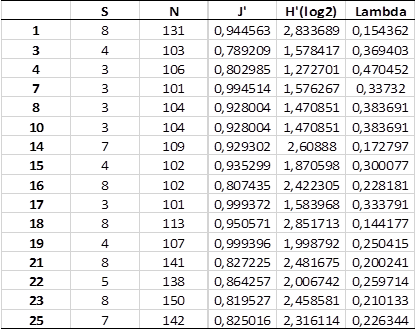
Table 5 Shows that number of species (S) varies between 3 (stations 7, 8, 10, 17) and 8 (1, 16, 18, 21, 23), the number of individuals (N) varies between 101 (station 7) and 150 (23), evenness (J) varied between 0.78 (3) and 0.99 (7, 17, 19), diversity (H´log2) varied between 1.27 (4) and 2.83 (1) and the dominance (lambda) varied between 0.14 (18) and 0.47 (4)
Cluster analysis based on foraminifera species, revealed that at the 60% similarity level, the stations form 5 groups (Figure 8) similar in terms of the ecology and are described below:

Figure 8 Cluster analysis based on foraminifera species, excluding sterile samples (2, 5, 6, 9, 11, 12, 13, 20, 24) forming five groups (a - e).
Group a: stations 7 and 17 (low number of species). Group b: 19 and 21 (high evenness and high number of species). Group c: 14, 18, 1 and 16 (high number of species). Group d: 25, 15 and 23 (high and low number of species). Group e: 22, 4, 3, 8 and 10 (low number of species, low diversity, high dominance).
Groups formed in the cluster analysis were evidenced by MDS analysis (Figure 9). Variation in the cluster occurred mainly because of diversity of species. Group (a) with stations with low number of species; group (c) with groups the stations with a high number of species.
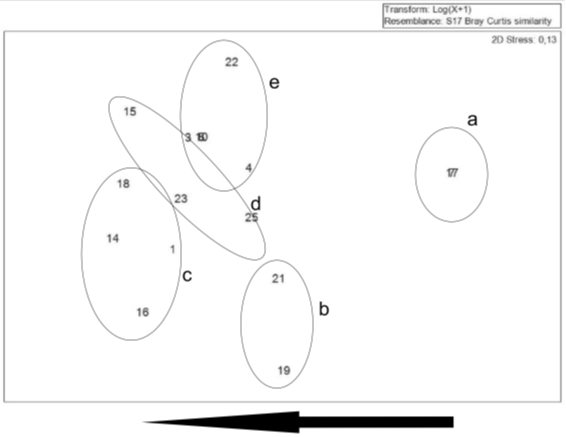
Figure 9 MDS analysis performed at stations containing foraminiferal species. Sterile samples were excluded. The arrow shows increase in diversity.
Our results point out the indicator species found in the study area and are illustrated in plate 1. Water temperature indicators: 1. Pseudononion atlanticum, 2. Elphidium sp., 3. Buccella peruviana, 4. Quinqueloculina patagonica. Freshwater indicators: 5. Miliammina fusca, 6. Miliolinella subrotunda. Indicators of water with medium turbidity: 7. Clavulina sp., 8. Quinqueloculina lamarckiana, 9. Reophax sp., 10. Bolivina striatula, 11. Textularia earlandi, 12. Triloculina trigonula. Indicators of transition environments and specific type of sediment (low sphericity) 13. Bulimina marginata, 14. Nonion sp., 15. Bolivina ordinaria.

Plate 1 Water temperature indicator: 1. Pseudononion atlanticum, 2. Elphidium sp., 3. Buccella peruviana, 4. Quinqueloculina patagonica. Freshwater indicators: 5. Miliammina fusca, 6. Miliolinella subrotunda. Medium turbidity water indicator: 7. Clavulina sp., 8. Quinqueloculina lamarckiana, 9. Reophax sp., 10. Bolivina striatula, 11. Textularia earlandi, 12. Triloculina trigonula. Transitional and low sphericity sediment indicator: 13. Bulimina marginata, 14. Nonion sp., 15. Bolivina ordinaria.
Here we point out an increasing gradient of pH, turbidity and mean particle size and a decreasing of magnetic susceptibility towards the northern area of study. The increase is probably due to the proximity to the Ibiraquera Lagoon bar, where the flow of fresh water occurs on the inner shelf, in an area of lower depths. Regarding salinity, we observed lower rates in the region of the slope that divides the harbor area and the north part of the area, a wet site with a large volume of runoff from surface freshwater, presenting the lowest values obtained in the sampling, even lower compared to the values near the exit of the Lagoa de Ibiraquera bar. The increase in water turbidity reduces chlorophyll values, revealing the availability of light as a limiting factor to primary productivity in the north of the region, probably due to a natural eutrophication process linked to the mouth of the Ibiraquera bar. It is possible to observe an area of maximum turbidity, typical of the mouth of the lagoon with estuarine conditions, in station 5 and the consequent gradient related to the supply of fresh water, coming from Ibiraquera Lagoon bar, forming a front of estuarine plume with distinction of at least two different water masses.
In general, study area shows higher salinity in the north and lower in the south. Surface temperature at the southern part, close to the harbor, presents higher temperature, and northern part has lower temperature. Water mass from southern enter the region through the north part of the study area, and the northern water masses penetrate the Ibiraquera Lagoon, however, they do not penetrate intensively due to the presence of the island of Batuta as a natural barrier and to the discharge of the waters in the Ibiraquera Lagoon.
Although data was collected in summer, a time of high rainfall, foraminifera represent an average of what occurs in the water column over the past year and show evidence of how these variables interact in the region where right whales visit every year. Colder waters from the South, which also reaches northern and central parts, influence the inner shelf near to Ribanceira and Ibiraquera.
A decrease in salinity variation is observed due to the influence of freshwater from the beach in the south and from the bar of the Ibiraquera Lagoon to the North, which form a hypo saline stagnated water with higher pH in the central region of the study area.
This dynamic structure formed by the process of discharging waters of continental origin near the coastal zone is a water body of a density that contrasts with the density of ocean waters, with high concentrations of suspended materials. Studies indicate the relationship between the occurrence of waters of continental origin and the presence of cohesive sediment banks in coastal areas.
The inner shelf is influenced by two important sources of freshwater of continental origin, the Ibiraquera bar and the beach in the south. It is therefore a region of great dynamic complexity, a terrigenous sediment with low salinity and high turbidity due to the influence of large freshwater contribution combined with winds and currents seasonality. The magnetic minerals have strongly positive magnetic susceptibility with continental origin and detritic (ferromagnetic) mainly transported by rivers. Other magnetic minerals have low magnetic susceptibility (paramagnetic) such as clays. The results point to the harbor region with the highest magnetic susceptibility values together with the finer particle size. Therefore, this means that these results are related to the presence of metals from harbor activity and/or more claylike materials, which have the capacity to retain greater amount of pollutants. Either way, harbors are built in low-energy sheltered regions. The detrital magnetic minerals enter the coast, are transported by coastal drift currents, and are deposited in the harbor region. This second factor is not directly related to the direct pollution of the port. The lowest values of magnetic susceptibility are shallow areas where sand predominates. There is decrease of magnetic susceptibility from south to north on the deep waters, which occurs deposition of finer materials in relation with the hydrodynamic region.
The natural environmental conditions of the sediment and water that were observed influence the benthic foraminifera, which again proved their importance as bioindicators in a bento-pelagic environment of extreme ecological importance. The interaction of benthic and pelagic organisms (foraminifera and whales, respectively, in this study) is known as bento-pelagic coupling.
Since depth controls vertical arrangement and morphology of species, because usually the greater the depth, the greater the size and quantity of shell ornamentation,31 reflecting the specificities of the habitat of these organisms. Our data on sterile fauna reflects differences in depth where availability of food (carbon flow) and dissolved oxygen, up to 7 m in depth, are different from other sampling stations located in higher depths, limiting foraminiferal distribution. Water depth and food availability are still associated with primary production on the surface and decomposing organic matter in the local sediment characteristic of a given associated water body. Based on this statement, it is concluded that the conditions of the isobates of up to 7 m differ from the sedimentary conditions and of water masses from around 10m deep.
Results indicate that Textularia earlandi, Triloculina trigonula, Clavulina sp., Quinqueloculina lamarckiana, Reophax sp ., and Bolivina striatula are found in medium turbidity, and in station 16, the increase in Textularia earlandi, Reophax sp. and Clavulina sp. can be attributed to type of sediment, since they are non-selective and choose grains of different sizes to compose their shells. A study by Kitazato suggests that foraminifera have particular preferences with regard to the substrate, which depend on the size and roundness of the grains, as well as on the sediment compaction. Textularia earlandi was found in Lagoa dos Patos by Boltovskoy and is characteristic of environments where salinity is greater than 25 and depth less than 30m, indicating estuarine areas, corroborating estuarine flow of the Ibiraquera Lagoon and the wet part of the beach in the south. The suborder Textulariina is not normally associated with warm water, probably due to competition with calcareous forms.32 Agglutinated forms are more abundant at low salinity, decrease in frequency when there is an increase in salinity33 and are found in places subject to higher discharge of fresh water, characteristic of brackish environments. The approximate size of the grains and the rapid compaction of the sediments, as they accumulate after the deposition of a turbidity flow, result in inadequate habitat conditions by the infaunal populations of Textularia earlandi which, perhaps for this reason, prefers environments with medium turbidity. Triloculina trigonula, a calacareous species with perforated wall, also seems to tolerate this inner shelf like estuarine environment. Quinqueloculina lamarckiana, Reophax sp., and Bolivina striatula show an increase in occurrence in station 17, where the average granulometry values are higher due to the ravines and gullies located on a slope with a high slope, where fresh water and erosion bring more coarse sediments and are less regular for the south part of the beach. Bolivina and Quinqueloculin are free forms and live inside or on soft substrates.32 Ribeiro Neto34 observed greater diversity in subsurface sediments of the continental shelf of the Abrolhos reef complex in southern Bahia, with a predominance of Quinqueloculina sp. characteristic of this particle size fraction.
Observations also point out that the region of medium turbidity has water with less salinity and roundness of the grains, however with greater diversity and evenness of species in the central part. In the southern part, we observed an increase in diversity with the appearance of Miliolinella subrotunda and Miliammina fusca. Romano et al.35 studying the relationship of grain size in the marine sediments of a highly impacted harbor in Sicily, Italy, observed a clear regression in the density and diversity of foraminifera, with the exception of Miliolinella subrotunda, which had an increase in dominance, in less polluted places. This species optionally builds a long flexible tube several millimeters above the sediment, which allows the organism to feed on particles in the stream just above the surface of the sediment, apparently with higher nutritional values and a greater amount of food.36
The northern and southern areas are more saline than the central part of the beach. Station 22 is one with the highest salinities. In addition, we observed that near station 24, where domestic sewage is being released less than 15m deep, diversity is low, with high dominance and evenness.
Since temperature is one of the most important factors in controlling the distribution of foraminifer, even the little variation in the study region was able to differentiate microhabitats. It is observed that the northern part has a colder temperature than the southern part and that an increase in Pseudononion atlanticum, Buccella peruviana, and Quinqueloculina patagonica occurs where the temperature is around 21 °C. It is important to point out the competition between Buccella peruviana and Quinqueloculina patagonica. One flourishes in the decrease and absence of the other, unravels interspecific competition, suggesting the ecological niche in this microhabitat. In Callao, Peru, Buccella peruviana has been described as being transported to the Atlantic by sub-Antarctic waters and Eichler et al.27 showed that this species is characteristic of Sub-Antarctic Platform Water (SASW) (T <15ºC and 33.7 <S <34.15) dominant mainly below Punta Del Diablo (33º S) in summer and winter. Stevenson et al. describe this species as from temperate water, typical of the Argentine province and concluded that the sub-Antarctic waters reached areas of lagoons located in the northern part of Rio de Janeiro (22º S), in winter. This species also occurs closer to the coast, and in Cabo de Santa Marta (28º S, Santa Catarina) it also occurs in the deepest stations (105 and 130 m), and was suggested by Eichler et al. as indicative of the cold-water of sub Antarctic origin, the Sub Antarctic Shelf Water (SASW), passively transported by the predominant currents towards north. In another approach, Eichler et al.27studying foraminifera as indicators of contamination of marine pollutants in the inner shelf of southern Brazil, inferred that the distribution of Buccella peruviana is being influenced mainly by temperature, depth, salinity and percentage of sand, and not by the percentage of clay and total coliforms. The present study points to Elphidium spp. as a the limit of marine waters, close to the mouth of the Ibiraquera Lagoon, corroborating what was also observed in the mouths of the Patos Lagoon and Plata River by Eichler.27 In addition, Eichler et al.22 found in the Bertioga channel the species Elphidium excavatum and Elphidium poeyanum in polyhaline waters (18-30) where sandy and silty sediments from the Bertioga estuarine channel are enriched with organic carbon and phosphorus, suggesting the reach of ocean water in this environment. Our results indicate that higher dominance occurs in the northern part of the study area, and in the southern part, close to the harbor, there are two types of sediment with different patterns: stations 24 and 1 with larger average sizes and greater deviation, a pattern close to the outfall with slightly higher dominance of species, and stations 23 and 25, which present smaller grain sizes and little or no sand near the Harbor. This study also points out that sphericity is lower in places where there is a greater amount of fresh water, in the central part and in stations 2 and 22, close to the domestic sewage outfall. It is also observed that lower sphericity values occur where values of higher grain size. Bulimina marginata and Nonion sp. occur in the central part of the study, where there is formation of hyposaline region. This region can be considered as a transition between three bodies of water: colder and salty water in the north, fresher and warmer in the south and saltier and medium temperature in the central part. These foraminiferal species are identified as tolerant to the transition region of fresh and salt water, warm and cold. Our data agrees with Eichler et al.,37 which described Bulimina marginata on the western continental margin of the South Atlantic dominated by three main water masses: Sub Antarctic Shelf Water (cold and salt water, SASW), Sub Tropical Shelf Water (warm, saline, STSW) and estuarine plume from Prata River (cold and fresher). Despite the great seasonal variability of the Plata River estuarine plume extension along the shelf, an intense and relatively stable temperature salinity gradient separates the SASW and STSW, forming the Subtropical Shelf Front (STSF) around 32°S. Our results show that the distribution of Nonion sp. and Bolivina ordinaria occurs in the same locations as Bulimina marginata.
Here we show foraminiferal species indicator of water temperature (around 21°C): Pseudononion atlanticum, Elphidium sp., Buccella peruviana, Quinqueloculina patagoniana; of low salinity: Miliammina fusca and Miliolinella subrotunda; of medium turbidity: Clavulina sp., Quinqueloculina lamarckiana, Reophax sp., Bolivina striatula, Textularia earlandi, Triloculina trigonula and of transition environments with specific sediment type (low sphericity): Bulimina marginata, Nonion sp., Bolivina ordinaria. BEST analysis points out that the variables that most influence foraminiferal species were depth, surface temperature and salinity, and that in the other hand sediment grain sizes, morphometry and magnetic susceptibility are the variables that least influence the distribution of these organisms.
We know that the Right whale, especially the pairs of female and calf, has preference for shallower areas with less than 10 meters deep7,38 and among other environmental variables, temperature of the water surface, proximity to freshwater discharges.24 The results show that shallower areas below 7 m, the high hydrodynamics of the surf area make it difficult for benthic foraminifera to attach to the sediment. The more saline northern part where species of foraminifera that indicate water temperature of 21°C (Pseudononion atlanticum, Elphidium sp., Buccella peruviana, Quinqueloculina patagonica) are well established is the preferred area for the presence of the mother and calf.
The harbor, with its higher temperatures, lower pH and turbidity values and higher depth due to dredging for harbor operations, does not seem to be favorable for the presence of Right whales for longer periods of time.
Conditions of the marine sediment at 5m differ from 10m deep, and Buccella peruviana and Quinqueloculina patagonica are competitors, one flourishes in the decrease and absence of the other. Bulimina marginata and Nonion sp. occur in the central part of the area of study and tolerate transition region between three water masses. Water temperature (around 21°C) is habitat for Pseudononion atlanticum, Elphidium sp, Buccella peruviana, Quinqueloculina patagonica, low salinity is habitat for Miliammina fusca and Miliolinella subrotunda, and water with medium turbidity is habitat for Clavulina sp., Quinqueloculina lamarckiana, Reophax sp., Bolivina striatula , Textularia earlandi, Triloculina trigonula. Transition environments with low sphericity of the sediment is habitat for Bulimina marginata, Nonion sp., and Bolivina ordinaria.
Our studies indicate that the northern part of the region where Pseudononion atlanticum, Elphidium sp. Buccella peruviana, and Quinqueloculina patagonica is more saline with temperature of 21°C water is an ideal permanence place for mother and calf pairs, whereas the Harbor with its higher temperatures, lower pH and turbidity values and higher depth due to dredging for harbor operations is not favorable for the presence of the Right whales for long periods.39–47
The authors are grateful to the Marine Geology and Geophysical and Environmental Monitoring Laboratories (Laboratório de Geologia e GeofísicaMarinha e Monitoramento Ambiental Coastal/GGEMMA), and to the Graduate Program in Geology and Geodynamics (PPGG) of the Federal University of Rio Grande do Norte (UFRN).
We are grateful to CAPES for funding support through Projects Special Visiting Professor (98/2017-05, PVE 151-2012, AuxPe 242-2013), and Ciências do Mar II (23038.004320/2014-1) that enabled a Post Doc fellowship for P.P.B. Eichler at Moss Landing Marine Laboratories of San Jose State University (MLML/SJSU), and at the Ocean Sciences Department of the University of California at Santa Cruz (UCSC) (88887.305531/2018- 00, 88881.188496/2018-01 and 9999.000098/2017-05).
We want to thank the Technical Support to Strengthen National Palaeontology (Apoio Técnico para Fortalecimento da Paleontologia Nacional, Ministério da Ciência e Tecnologia MCTI/National Research Council CNPq Nº 23/2011, Nº 552976/2011-3).
This work was carried out under the scope of INCT Amb Tropic phase II (CNPq Process 465634/2014 1) linked to the Emergency Action to combat the 2019 oils pill of the MCTI (DTI-A 381360/2020-2 fellowship to PPB Eichler). We would like to thank the São Paulo State Research Support Foundation (FAPESP Proc. 96/4191-8, 98/05409-2, 99/10678-5). We are grateful to EcoLogicProject by facilitating the execution of the work.
Authors declares there are no conflicts of interests.

©2022 Eichler, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.