Journal of
eISSN: 2378-3184


Research Article Volume 5 Issue 2
1Lagoon Ecology and Aquaculture Laboratory (LEALab), Italy
2Institute for Environmental Protection and Research (ISPRA), Italy
3Technical Department Civil Engineering and Marine Works, Tuscany Region, Italy
Correspondence: Mauro Lenzi, Lagoon Ecology and Aquaculture Laboratory (LEALab), via G. Leopardi 9, 58015 Orbetello, Italy
Received: December 23, 2016 | Published: February 8, 2017
Citation: Lenzi M, Persiano ML, Gennaro P, Rubegni F (2017) Artificial Top Layer Sediment Resuspension To Counteract Chaetomorpha Linum (Muller) Kutz Blooms In A Eutrophic Lagoon Three Years Full-Scale Experience. J Aquac Mar Biol 5(2): 0 DOI: 10.15406/jamb.2017.05.00114
This study analyzes the full-scale results of a three-year eutrophication control and management in the Orbetello lagoon. We examined the cause/effect relationship among the resuspension of top layer sediment in the water column (carried out by specifically designated boats), the labile organic matter present in the sediments (LOM), and the values of Chaetomorpha linum high density mats, which insist in the disturbed areas. LOM values resulted inversely associated to the frequency of sediment disturbance. LOM decrease, together with higher oxidation levels, triggered the reduction of nutrient release with an immediate effect on algal masses: the decay of the mat under layer prevailed on the growth of the mat over layer. Conversely, with a reduced frequency of disturbance, and the consequent increase of both the LOM as well as the anaerobic processes of the sediment which promote nutrient releases, the algal growth showed a time lag of several months, in which the nutrients uptake was likely intense. The stored nutrients were then used only some months later, when the environmental conditions became favorable for the surprisingly rapid and impressive development of the mats.
Keywords: Chaetomorpha linum, Macroalgal mat, Lagoon sediment, Eutrophication, Sediment disturbance, Labile organic matter
SAT, Surface Air Temperature; SSS, Sea Surface Salinity; SST, Sea surface temperature; RH, Relative Humidity; DO, Dissolved Oxygen; BOB, Bay of Bengal; IO, Indian Ocean
In the last forty years, coastal areas have been increasingly subjected to the consequences of eutrophication, which is shown by the opportunistic developments of microphyte and macroalgae.1-3 Such developments can be very intense (>30 mg chl m-3, for the first, and >10 kg more serious m-2, for the second) and produce consequences even more serious as a result of their decay, such as drifts of environmental deterioration, which are remarkably evident, above all, in the case of Mediterranean non tidal lagoons. These have poor water exchange by their structural feature and tend to accumulate energy in the system, mainly in terms of sedimentary organic detritus, as it occurs, e.g., in the lagoons of Prevost,4 Thau,5 Cabras,6 and Orbetello.7 The remineralization of the bloom after its collapse is conducted mainly by the sulfate reducing bacteria, and produces hypoxic or anoxic conditions and sulphides accumulation, which constitute the final stage of the summer environmental crises of these environments, causing dystrophies, and sometimes die-offs of aquatic fauna.7-9
Several measures, acting at different ecological levels, have been proposed to overcome this problem in such environments. The most rational solution would be to eliminate the nutrient source. Therefore, solutions involving the collection and treatment of urban wastewaters and measures to limit the input of urban, agricultural and industrial wastewaters are often proposed and, occasionally, also interventions to limit urban development and to modify agricultural practices.10-12 However, this kind of approach is not always feasible or suitable like in the case of densely populated areas. A an alternative, more emphasis has been placed on heavy interventions that modify basin hydrology through sea-communicating canals, underwater canals, changes in internal shoreline morphology, water control in the hydrographic basin, massive removal of sediments as the most important source of nutrient release after decades of nutritional supplements, or as water replacement through the pumping of sea water.10,12-22
Because of the complexity of the settlements, that in many cases surround these coastal areas, and the engineering works and their impact and management costs, these types of recovery interventions are not always achievable. Moreover, because of the laminar condition of the basins, large areas are sidelined to the water renewal or to the effects of artificial morphological changes, and it is unlikely that a sufficient water renewal is reached in much of the basin such as to prevent the summer dominance of the anaerobic bacteria activity. The forced water exchange and submerged canals may therefore be useless when they are devised in low-energy systems. Submerged canals may even be harmful, resulting in stagnant ditches, receptacles of organic matter.
Other solutions may act downstream, either at the level of macroalgal mass, or at sediment level. In the first case, the macroalgal mass is removed in order to avoid the consequences of its decay,14,23,24,25 while, for the latter, the reduction of organic accumulation is attempted by forcing an oxygenic mineralization through its resuspension in the water column.26-29
The aim of this study is to report and discuss the results of a three-years-long, full-scale eutrophication management in the Orbetello lagoon by resuspension of the top layer sediment in the water column, taking into account the percentage of labile organic matter in the sediment, and the biomass of Chaetomorpha linum high density mats.
Introductory framework of the study area
The Orbetello lagoon is a shallow, non-tidal, eutrophic environment with low water-turnover. It is located along the Tyrrhenian coast (42°25’–42°29’ N; 11°10’–11°17’ E) and consists of two communicating basins (i.e. western and eastern) of 15.25 km2 and 10.00 km2 (Figure 1). The lagoon is highly eutrophic because of land-based fish-farms wastewaters, intermittent agricultural run-off flow and nutritional sediment stores coming from the urban and productive activities wastewaters discharged in the past.30 In 1994, following a prolonged environmental crisis that heavily damaged fishery and tourism, the lagoon management was entrusted to a Government Authority, which succeeded in removing part of the nutrient inputs, building submerged canals, strengthening seawater pumping stations and increasing the harvesting and removal of some of algal masses. Since 2005, after a first improvement of the environmental conditions led to the return of seagrass,30 the conditions of the lagoon worsened again and showed important macroalgal developments, despite the efforts of the Authority. In 2013, the Tuscany Region took over the environmental management of the lagoon and, in 2014, a new three-year plan was drawn. This plan adopted the sediment resuspension method in an area of the western basin of 400 hectares (Figure 1) affected by a persistent, intense macroalgal bloom, dominated by Chlorophycea Chaetomorpha linum (Müller) Kütz.
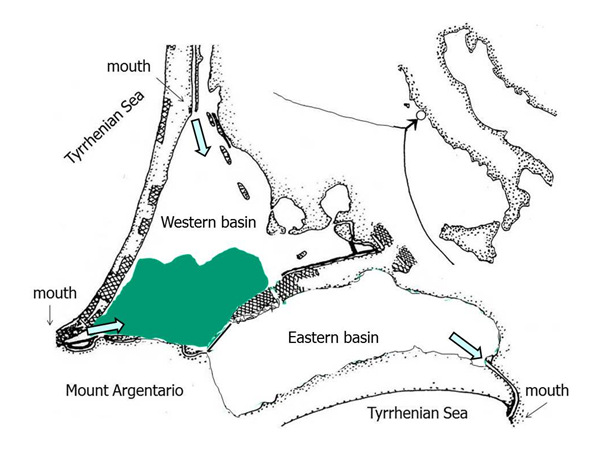
Figure 1 Orbetello lagoon along the southern coast of Tuscany, Italy. Reticulated areas: settlements; arrows: inflow and outflow of pump strokes marine waters; colored surface: operative area with macroalgal high density mats.
Some considerations on macroalgal harvesting and sea-water pumping
Macroalgal harvesting and sea-water pumping were the first actions taken to counteract the effects of eutrophication.14 The harvest took place in a relevant measure since 1994, and was carried out with four boats able to collect and carry 2 ton of alga per travel. The harvest was done for 6 months per year, and took away from 4000 to 6000 tons of wet algal mass (estimated value at 24 h of rest).30
The pumping consisted of the intake of sea-water by two water-scooping stations situated in the western basin, for a total of 12 m3 s-1, and in its flow through the sea mouth of the eastern basin (Figure 1). This activity was carried out between June and September.
Both the devices have failed to deal effectively with 1) the development of algae, 2) the accumulations of sedimentary organic detritus, 3) the summer temperatures reached by the water body and, essentially, 4) with the prevention of important and widespread sulfate-reducing bacteria activities, with the consequences that this may entail. Furthermore, from evaluations carried out in the field [31, submitted], the growth of Chaetomorpha linum, the species largely dominant, occurs mainly in the upper layer (about 20cm) of the algal mat thickness, with an average daily growth of 5%. Therefore, a high density mat as the one of June 2014, covering 400 hectares, with an average density of 10 kg m-2 and thickness of 80 cm, could have produced every day about 500 tons. And this in light of 30-35 tons being removed every working day from the four boats, equipped for this purpose.
Finally, regarding the sea-water pumping, it must be said that the laminar nature of the basin (an area of 25 km2 and an average depth of 1 m) has never allowed a sufficient water exchange to prevent neither large areas from stagnation -the water pumped tends to flow along paths in a low energy, with a speed that does not exceed 1-1.5 cms-1 nor remarkable improvement of the environmental conditions.32 The hydrodynamic of the basin remains under the total control of the winds.33,34
Conceptual models of the high density macroalgal mat growth and decay, and sediment resuspension
If the Chaetomorpha linum mat grows within about the first 20 cm, the underlying part does not carry out photosynthesis and is subject to bacterial attacks,35 to the point that the last decimeter of the mat thickness decays and produces organic mud on the bottom, enriching the sediment in carbon and nitrogen36 and producing effects on microbial community and processes.37 The anaerobic mineralization that occurs at the sediment–water interface releases inorganic nutrients toward the water column,38 such as ammoniun (NH4+),39 and carbon dioxide (CO2) and orthophosphate (PO43-).40 Phosphorus release occurs mainly in the anoxic degradation of organic detritus.41 Oxygen and pH decreases lead to the reduction of the Fe3+ which involves the breaking of the bond of PO43- with the ferric oxides-hydroxides,42 and subsequently its release into the water column.43 This supports further growth of the mat overlayer, but produces also soluble and volatile sulfides (H2S, HS-, S2-; SVS) by the sulfate reduction bacteria activity, which can accelerate the degradation of the mat under layer.44 Therefore, decomposing macroalgae is an important nutrient source. The mat is thus self-sustaining in a continuum, and can increase or decrease in density in relation to the prevalence of the activity of its over layer or that of the bacteria on the under layer. Moreover, in the mat over-layer, where macroalgal growth is highest, N and P availability from upward diffusion of benthic flux is low due to under-layer macroalgal uptake. Nutrients must be obtained from the water column, causing a downward flux of nutrients from surface water into the mat over-layer.45 Under these conditions, paradoxically, dissolved nutrients analyses provide very often an oligotrophic framework, and are not reliable to determine the actual trophic state of the basin and nutritional bioavailability. In this regard, in C. linum high density mat, Lenzi et al.46 observed stratifications of nutrients dissolved with relatively higher values on the bottom of N-NH4, dissolved organic nitrogen, and orthophosphate, while dissolved inorganic nitrogen:orthophosphate atomic ratio was decreasing downward. Moreover, they still observed significant tissue nutrient stratification: a greater accumulation of N and P in the under-layer thalli than in over-layer.
A frequent resuspension of anoxic sediments covered by the mat 1) blocks the activity of sulfate-reducing bacteria, likely because it carries the surficial sediments in areas where oxygen is present,27 2) enriches the detritus of aerobic bacteria located in the water column, pushing for aerobic mineralization,47 3) tends to favour the processes of nitrification/denitrification,48-50 4) produces conditions for which the orthophosphates are blocked through bonds with the ferric oxy-hydroxides,42 creating phosphorus limitation for the mat, and, ultimately, 5) reduces the load of sediment labile organic matter (LOM),51,26,27 bringing the sediment into "security conditions", for a short/medium duration, with respect to the onset of dystrophic phenomena. For a review, see Lenzi.52 Finally, Lenzi et al.26,27,28 did not observe a significant nutrients increase in the water column during the sediment resuspension activities.
Three-years environmental management plan by sediment resuspension
Sediment resuspension policy was adopted by the Tuscany Region Government in the Orbetello lagoon (Italy) in the period 2014-16. This three-year plan of environmental management, set to the sediment resuspension, included an intervention of 4 boats. Taking into account the modest depth of the Orbetello lagoon basins, resuspension has been obtained for the simple repeated passage of the boats previously used for the algal harvesting, in the operative area (Figure 1). In fact, the boat tonnage was sufficient to affect the soft surface sediments through the thrust on the shifted water mass, for 2-4 cm,53 though, more recently, for improving the efficiency in the zones at greater depth, the same boats have been equipped with insufflators, that send air towards the bottom with the pressure of a compressor.
The boats sailed in the operative area of 400 hectares affected by high density macroalgal mat, for the daily duty of 7 hours, for 24 days a month, for 18 months spread over a period of 27 months. The boats were designed to operate daily in a sub-sector of 25-30 hectares in order to cover the entire surface, and were directed in those areas where the LOM had the highest values, according to the monitoring. The activity was conducted mainly in autumn and winter, when the oxygenic mineralization of organic load resuspended would have been more effective. However, in the summer critical period, the use of boats has sometimes been employed with the aim of blocking the activity of the sulfate-reducing bacteria that occurs when the anoxic sediments are in contact with the oxygen of the water column. This last phenomenon reduces the production of soluble sulfides and consequently the risk of dystrophic crisis.
The monitoring consisted essentially in the estimation of the macroalgal biomass and of the sediment organic matter. In this study, we did not conduct dissolved nutrients analyses, assuming the picture that emerges from the aforementioned studies on the characteristics produced by high density macroalgal mat and the effects of sediment resuspension.
Macroalgal biomass estimates
Macroalgal mass estimates were conducted between June 2014 and September 2016, by georeferencing and dividing the operational area of 400 hectares (Figure 1) into 4 sectors, subsequently divided into sub-sectors of 25-30 hectares identified by color flag, to facilitate the field operations of monitoring and boats. Biomass estimates (b, kg wet weight m-2) were mainly conducted bimonthly by 45 samples per trial collected using a square metal frame of 60x60 cm. Three sampling stations were randomly chosen along a diagonal transect for each sub-sector, discarding the bare bottom areas when these were eventually found. The algal mat density was therefore assessed in relation to the only vegetated area, while the bare bottom areas have been considered by evaluating the total cover of the substrate (CT) by vegetation, expressed as covered surface to total surface ratio for each station area.54 The material collected inside the square metal frame has been drained for a few minutes and weighed directly in the field with portable electronic balance with sensitivity ±0.5 g, and classified as surface area unit of 1m2 (factor processing 2.778). Finally, standing crop (SC; i.e., the algal mass present in a given lagoon surface at the time of survey) was calculated using the following equation:
SC = b * CT * 10-3 * A
where b and CT result from the average of all the estimated values, A is the surface in m2 of the total operative area, and 10-3 is the conversion factor for kg into tons, as standing crop was expressed in tons wet weight (TWW).
Organic matter determination
30 sediment samples for trial (bimonthly or on a quarterly basis) were collected in homogeneously distributed points inside the operative area (two per each sub-sector), in the upper 3 cm using a horizontal sampler with 60 mL syringe, lowered from the boat and guided by an appropriate device.29 Samples were frozen until analysis, when they were wet sieved with fresh water (1 mm mesh), dried at 75°C for 48 h and weighed. Labile organic matter (LOM) was determined as loss of weight after combustion in a muffle furnace for 6 h at 250°C.55
Statistical analysis
Differences in LOM% across different dataset were calculated with Student’s t- test and illustrated through a scatter plot, using the Statistica 10 software package.
Macroalgal biomass
In Table 1, biomass (b; mean ± SD) and standing crop (SC) calculated for the total area of about 400 hectares affected by high densitymacroalgal mat, are reported for the various surveys. In Figure 2, the SCs estimated during 2014-2016 are compared with those of previous 4 years, in which the environmental management was based by macroalgal harvesting (Lenzi, unpubl. data).
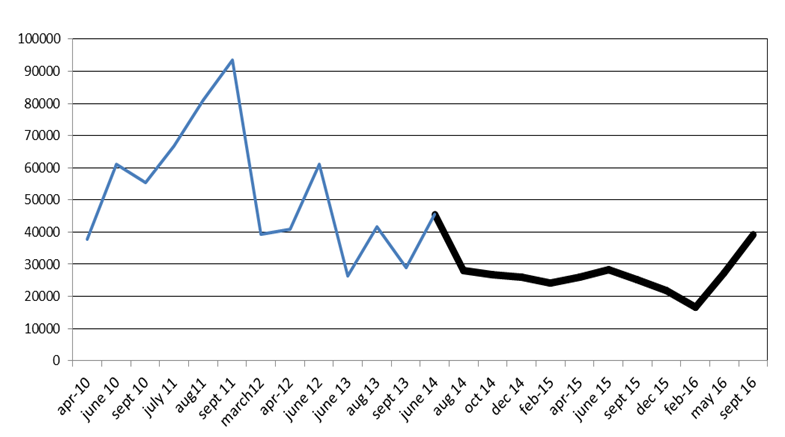
Figure 2 Macroalgal standing crops in ton wet weight of the Orbetello lagoon estimates between April 2010 and September 2016. In bold, the period when it was operated with the resuspension of sediments.
In June 2014, when the sediment resuspension method began, the mat density was relatively high, with a SC of 45,447 tons, and b of 10.98 ± 5.59 kg m-2, with the latter pretty well uniformly distributed. Afterwards, the values of b and SC dropped and the last remained fairly stable up to February 2016 on an average of 25,032 ± 3,297 tons, with a loss of -38.4% already in August 2014.
The series of estimates reported in Table 1 shows two relative peaks beyond the initial peak, which correspond to the moment of greatest algal growth, between May and June. The SC increase that took place in May 2016 was the most important increase registered, as it accounted for 63.1%, referring to the immediately preceding estimate. This trend continued throughout the estimate of September 2016, which yields an increase of 43% compared to the May estimate.
|
B |
SC |
LOM |
F |
LOM% |
||
|
kg m-2 |
ton |
% |
for period |
|||
|
2014 |
June |
10.98 ± 5.59 |
45447 |
20.78 ± 3.33 |
26 |
|
|
July |
100 |
first |
||||
|
Aug |
6.09 ± 3.40 |
27981 |
8.46 ± 2.09 |
93 |
||
|
Sept |
104 |
|||||
|
Oct |
6.98 ± 3.65 |
26855 |
9.23 ± 2.96 |
105 |
||
|
Nov |
76 |
|||||
|
Dec |
6.89 ± 4.31 |
25862 |
8.22 ± 2.54 |
53 |
||
|
2015 |
Jan |
61 |
||||
|
Feb |
6.50 ± 4.32 |
24098 |
7.74 ± 1.12 |
76 |
||
|
March |
60 |
|||||
|
Apr |
7.35 ± 4.15 |
26002 |
8,3 ± 1.64 |
23 |
||
|
May |
25 |
f 816 |
||||
|
June |
7.59 ± 4.51 |
28235 |
8.79 ± 2.54 |
14 |
8.40 ± 2.37 |
|
|
July |
67 |
second |
||||
|
Aug |
59 |
|||||
|
Sept |
6.97 ± 3.85 |
25356 |
12.03 ± 9.78 |
0 |
||
|
Oct |
41 |
|||||
|
Nov |
43 |
|||||
|
Dec |
6.13 ± 4.12 |
21879 |
11.75 ± 4.82 |
46 |
||
|
2016 |
Jan |
33 |
f 309 |
|||
|
Feb |
4.64 ± 4.04 |
16744 |
13.06 ± 12.11 |
20 |
12.22 ± 9.46 |
|
|
March |
91 |
third |
||||
|
Apr |
100 |
|||||
|
May |
7.48 ± 3.87 |
27310 |
9.92 ± 5.94 |
40 |
f 231 |
|
|
June |
0 |
|||||
|
July |
29 |
|||||
|
Aug |
22 |
|||||
|
Sept |
9.99 ± 4.27 |
39043 |
7.39 ± 1.65 |
0 |
Table 1 Standing crops (SC, in ton wet weight) and biomass (b, in kg m-2 wet weight ± SD) of macroalgal high density mats, and sediment percentage of the labile organic matter (LOM ± SD %) collected under the macroalgal high density mats of the Orbetello lagoon, carried out between June 2014 and September 2016. f, the number of the boats interventions for each month; each unit corresponds to the daily interventions of a boat in a sub-sector of 25-30 hectares. In shades of gray, the month periods with different features of the LOM and SC, and LOM% (± SD) as the average of all the values of each period, and f as the sum of interventions for each period.
Labile organic matter
In Table1, sediment LOM percentages (mean ± SD) estimated in the operative area of 400 hectares, are reported for all trials. The initial values estimated in June 2014 were very high, but they declined sharply since August 2014, when a loss of 59.3% was recorded, and remained substantially stable up to June 2015. Up to that date, the values were fairly uniformly distributed, both in high initial values of June 2014 as well as in successive low values SD (Table 1; Figure 3). For the first 12 months, between July 2014 and June 2015 (first period), the average LOM between all the records was of 8.40 ± 2.37 % (Table 1), showing how this variable remained relatively stable and low throughout this period, and was fairly uniformly distributed on the bottom layers. In the following eight months, between July 2015 and February 2016 (second period), LOM values increased with an average of 12.22 ± 9.46 % (average among all the records; Table 1). The values resulted lowered again during the subsequent third period of three months (March-May 2016), and ending in September 2016 (Table 1). At t-test analysis for pairs of months (Table 2), it turns out that the data for August 2014 and those for October 2014 did not differ significantly from those for September 2015, as well as those for June 2015 and May 2016. Conversely, LOM data of February 2015 were significantly different from those of February 2016, and those of December 2014 compared the ones of December 2015.
|
Aug 14 vs Sept 15 |
8.46 ± 2.09 vs 12.03 ± 9.78 |
P = 0.056 |
|
Oct 14 vs Sept 15 |
9.23 ± 2.96 vs 12.03 ± 9.78 |
P = 0.184 |
|
Dec 14 vs Dec 15 |
8.22 ± 2.54 vs 11.75 ± 4.82 |
P = 0.001 |
|
Feb 15 vs Feb 16 |
7.74 ± 1.12 vs 13.06 ± 12.11 |
P = 0.025 |
|
Jun 15 vs May 16 |
8.79 ± 2.54 vs 9.92 ± 5.94 |
P = 0.312 |
Table 2 Results of the Student’s t-test comparisons among different pairs of months across the three-years study. Significant differences are reported in bold
The scatter plot of Figure 3 shows the LOM data dispersion along the vertical axis for each trial. It confirms 1) the uniqueness of June 2014 compared to all other trials, with the data showing above the regression curve, 2) the greater homogeneity of the data and their relatively lower values, more concentrated and mostly below the curve, between December 2014 and June 2015, the second part of the first period, and 3) the increase and a greater data dispersion with many records above the curve in the following periods.
Boats management
In Table 1, the total number of working days for all the available boats is reported on a monthly basis, and for the three different periods of boat interventions. Each unit of the number of working days corresponds to the daily action of the boat in a sub-sector. Since April 2015, the use of two boats has been lost, due to various breakdown never repaired; this was tartly compensated with the increasing shifts with the other two boats available.
LOM decrease happened in the first period, while for the mats the decrease continued throughout the second period. Therefore the subsequent increases of these variables showed a lag. In fact, LOM began to increase from September 2015, although far from the initial values of June 2014, and was highly variable until May 2016, conversely, the mat began to increase again from May 2016 (Table 1, Figures 2&3).
Regarding the LOM, the cause of the increase could be found in a good measure in the reduction of the boats activity between June 2015 and February 2016. This made the covering of the entire surface with the sediment resuspension more difficult, impeding the performance of the necessary repetitiveness of the process.29 In fact, the boat interventions from the end of June 2014 to June 2015 (first period) were 816, while from July 2015 to February 2016 (second period) the interventions were only 309 (Table 1). Pair wise comparisons between the December 2014 and December 2015 data-sets, and between the February 2015 and February 2016 data-sets showed significant differences in terms of LOM values (Table 2), suggesting that the increase of this variable in the second period was substantial, compared to the first period.
These data-sets seem to support the hypothesis of a direct effect of the action of the boats on LOM, even if the confront not significant between the datasets of August 2014 and October 2014 with September 2015 (Table 2) leaves identify a first half of the first period in which the LOM values still showed wide variability. That means the boat interventions must be extended in time to achieve the wished effects.
We can exclude that LOM reductions in the first 12 months may have been caused remarkably by natural processes, such as: 1) the strong winds capable of moving the soft sediments, as observed at other times in other areas of the same lagoon,34 because the areas covered by thick macroalgal mat are hardly affected by sediment resuspension, or 2) the activity of sulfate-reducing bacteria, as both periods (the first 12 months, and the second 8 months) had warm summers, the second period, in particular, in which this bacterial activity might have highly risen.
The LOM significant increase between in the second period was hence likely the result of the reduction of the disturbance activities due to faults which lowered the number of operating boats. To confirm this, an increase in the activity of the remaining two boats, 231 interventions in the three months between March and May 2016 (third period) through long shifts, corresponded to a new reduction of the LOM (Table 1). This reduction is comparable to the data set of June 2015 (t-test comparisons are in fact not significant; Table 2). Thus, the summer 2015, and that of 2016, at the end, albeit with difficulties, were addressed with relatively low LOM values.
Finally, a fourth period can be identified between June 2016 and September 2016. This was characterized by low LOM values which could be the result of both the intense boat activity in the third period, as boat interventions during the fourth period (four months) were relatively poor (Table 1), and of a possible minor decomposition of the mat under layer, as the SC values were dramatically reduced in February 2016, and the decomposition- with the consequent LOM production is higher the greater the density of the mat.
Data-sets also support the mat decrease that in the first period was likely due to the decrease of the nutrient release, linked to a reduced amount of LOM; but there is not a direct relationship between disturbance and macroalgal SCs decrease in the second period. This reduction of the mat might have been affected by the fragmentation of the algae by the propellers of the boats,56 but this action was likely proportionally present in both periods, as the algal mat continued to decrease in its biomass and standing crop until it reached its lowest value, estimated in February 2016, when the action of the boats was sensibly reduced (Table 1; Figure 2).
Why so long a lag between cause and effect regarding the mats? On the one hand, during the second period in which the LOM increased, there were 2 exceptionally hot months (30-34°C) in which the algal growth stopped, and 5 months, autumn and winter, relatively cold and, above all, with low photoperiod. In such conditions, the underlayer mat decay prevailing on the overlayer growth. On the other hand, C. linum has the property of accumulating nutrients when they become available,57 especially phosphorus, of which this Chlorophyta can more easily be subject to limitation,58 and use them at the moment propitious for growth. So the likely nutritional releases occurred in the period in which the LOM increased may have been the subject of intense uptake by the green alga. This would explain the surprisingly rapid and impressive developments of the mat with the onset of favorable season, estimated in May 2016 and in the months after.
On these bases, the oxygenic mineralization of LOM induced by the top layer sediment resuspension, should also result in an indirect decline in the mat biomass, which reduces its growth in the overlayer, while continuing its decay in the underlayer, if the action of the boats is very widespread and continued. We can hence assume that the resuspension process produced the prevalence of aerobic conditions in the sediment in the first and third periods, and caused a reduced availability of nutrients. In the second period, when the disturbance started declining, and the sediments began to get LOM rich again-more than they would have in case of greater intensity sediment disturbance-, the mat began to store the nutrient once again available. The biomass again increased, with a lag of more than 8 months, with the overlayer growth prevailing on the underlayer decay.
Comparing macroalgal SC estimated from 2010 to 2013 with those estimated in the three years 2014-2016 (Figure 2), led to the observation of a typical succession of peaks and declines for the former, meaning impressive spring growths and variable subsequent decay periods, and of a quite stable trend during 20 months of the study period. The decay that naturally occurred until 2013, was certainly in strictly anaerobic conditions, with a more intense and manifest soluble and volatile sulfides production, as spring and summer resulted warm. This type of decay predisposes to subsequent growth, releasing nutrients, especially orthophosphate, and it is extremely bad for the ecosystem, even when it does not produce obvious die-offs of wildlife.
We thank the Tuscany Region Government for making this study possible, the Orbetello Pesca Lagunare Company for the logistical support, and the Dr. Davide Risso for his contribution in the revision of the text.
None.

©2017 Lenzi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.