Journal of
eISSN: 2378-3184


The effect of boring sponge infestation on histology and ultra structure of mantle tissue in Perna indica was studied. Transmission Electron Microscopy (TEM) of the mantle tissue infested by boring sponges indicates that they cause irreparable damage to the cell organelles. The variations in the anatomy of the various cell organelles are discussed. Increased cellular autophagy, haemocytosis, hypertrophy of nucleus and endoplasmic reticulum, vesicle formation in nucleolus and whorl formation of mitochondria are noted in sponge infested specimens. The most consistent change was dystrophy of mantle in ultra thin sections. Sponge infested tissues also exhibited histopathological changes like degeneration of the mantle and infiltration of haemocytes.
Keywords:Histology, Ultra structure of mantle tissue, Perna indica, Boring sponges
Bubel1 described the fine structure of basal cells at the base of the periostracal groove of some marine bivalves. He also examined the cells lining the periostracum groove in some marine bivalves by means of electron microscopic studies.2 Gulka & Chang3 investigated on the prokaryotic infestation associated with the mass mortality of the sea scallop Plactopectenmagellanicus. Dix4 highlighted the role of mantle and pearl sac in pearl formation of the pearl oyster, Pinctadamaxima. The occurrence, prevalence, seasonality and histopathological progression of cellular disorder in Mytilusedulis from Yaquina Bay were studied by Mix.5 Kagoo & Ayyakkannu6 investigated on the pathological changes caused by parasites in Chicoreusramosus. Morrison7 described the structure of mantle and mantle lobes of Crassostreavirginica using both light and scanning electron microscopy. Zahab et al.8 studied the edge of the mantle in the pearl oyster Pinctada margaritifera. The organization of the mantle edge and the fine structure of the mantle epithelia of the neopilinid limpet, Laevipilinaantarctica were described by means of transmission electron microscopy.9 A further study of the cells of the inner face of the outer fold on M. edulis was also undertaken during periostracum repair.10
The method described by Old11 was followed for identification of sponge’s species infesting brown mussel. Two species were identified and they are Cliona lobata and Cliona vastifica. The classification of the species is as follows:
The mussels brought ashore regularly by the mussel pickers (skin-divers) in the morning hours were mainly utilized in the present study. A minimum of 100 shells was randomly sampled each time and in centers where more than one mussel picking units are involved, the samples were collected from each unit proportionately to make the total number 100. While collecting the specimens special care was taken to collect all size groups present in the landings. For extracting the spicules a bit of shell infested by sponge was placed in a test tube and concentrated nitric acid was poured into it and then heated over a low flame till the calcareous shell is completely dissolved. The test tube is then filled with water; stirred thoroughly and kept aside to allow the spicules to settle down. The supernatant water was then poured out gently and replaced with clean water. This process is continued for about 3-4 times. The residue thus obtained is a "spicule concentrate" and is almost free from nitric acid particles. One or two drops of the same were transferred to a micro slide by means of a fine dropper, spread it evenly and then mounted with water as the medium. Several drops were examined under different combination of lenses and necessary drawings were prepared using a camera lucida and species were identified.
In view of the frequent occurrence of bioeroding sponges in bivalves, histological and ultra structure studies were undertaken in Perna indica. The mantle epithelium just opposite to the pores through which the sponge papillae project out was separated carefully and used to investigate tissue level changes in the mantle. Sections of normal tissues were taken and compared with those of infested ones. Marginal zone of the mantle tissue was processed to study the histological manifestations of boring sponge infestation in brown mussel. The tissues were fixed immediately after dissection in Bouin’s fixative (ABF), embedded in paraffin wax and sectioned at 7 mm. The sections were deparaffinised, dehydrated, and stained with haematoxylene and eosin.12 The method described by Robinson et al.13 was followed for processing of tissues from the outer fold of the marginal zone of mantle for transmission electron microscopic studies. The tissues were fixed in 3% ice-cold glutaraldehyde for three hours. Three washes were given each in phosphate buffer (pH 7.2) to dissolve glutaraldehyde and post fixed 1% osmium tetroxide. The tissues were thoroughly washed in 0.1 M sodium cacodylate buffer and embedded in 1.0 % aqueous uranyl acetate in water for 1.5 hour till polymerization completed. Ultra thin sections were stained with lead citrate, dried, and observed under Hitachi 600 Philips CM 10 electron microscope.
On spicule preparation two species of boring sponges were identified from sponge infested brown mussel tissues; Cliona lobata and Cliona vastifica. Histopathological examinations of the infested tissues sectioned (7 mm) revealed the following changes in tissue morphology. The histological structure of mantle revealed the presence of three zones, and outer marginal zone with three folds, a pallial zone and a central zone. A single layer of stratified columnar epithelial cells of 40-50 mm thickness was present in this zone. The inner and outer surfaces were characterized by numerous mucous cells. The inner epithelium possessed dense cilia, large secretory cells, melanin pigment and deeply stained basal ovoid nuclei. In uninfested tissue the periostracum was visible as a thin yellowish fold (Plate 1, Figure A). The secretion of periostracal material was affected in sponge infested tissues (Plate 1, Figure B). The periostracum forms a thin sheet that extends out over the inner surface of the outer lobe which is covered with secretory granules.7 In normal tissue only few haemocytes were present in the marginal zone of mantle (Plate 1, Figure C). Haemocytosis was observed in infested tissues (Plate 1, Figure D & E). Other tissue anomalies include sloughing of the outer epithelial layer and increased secretion of wandering secretory cells. Another notable observation was vacuolization of various degrees in the mantle epithelium.
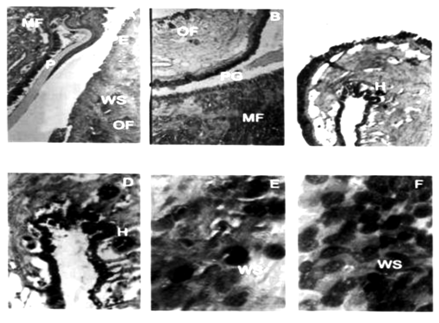
Plate 1 A-F Light Micrographs of mantle tissue of Perna indica.
A: Marginal Zone of Normal Mantle (C.S, H & E, X 200); PE: Pigmented Epithelium; WS: Wandering Secretory
Cells; OF: Outer Fold; MF: Middle Fold; P: Periostracum
B: Marginal Zone of Sponge Infested Mantle (C.S, H & E, X 200); PG: Periostracal Groove
C & D: Outer Fold Normal Mantle (OF) (C.S, H & E, X 200); H: Haemocyte; E&F: Outer Fold of Sponge Infested Mantle (OF) (C.S, H & E, X 200)
During the initial stages of shell damage increased activity of lysosomes was observed in the infested mantle cells. As infestation proceeds, cellular autophagy increased and the intensity of autophagy depends on the extent of sponge infestation. The organelles like nucleus, mitochondria and endoplasmic reticulum underwent rapid degradation possible through the action of lysosomal enzymes leading to cellular dystrophy. Electron micrographs of cytoplasm of the mantle tissue of uninfested mussel possessed nucleus with prominent chromatin granules (Plate 2, Figure A), endoplasmic reticulum, (Plate 2, Figure C), endocytic canals and mitochondria (Plate 2, Figure B). Initially, after the periostracum was slit by sponge boring, increased secretory activity occurs in the mantle. The most conspicuous change in the cytoplasm of the infested tissues was the presence of electron dense bodies (Plate 3, Figures B ,C) and fragmented endoplasmic reticulum (Plate 3 Figure A). Whorl formation (Plate 4, Figure A) and engulfing process of mitochondria (Plate 4, Figure B) were also observed in the infested tissues subjected to shell damage. Cytopathologic changes in the outer mantle tissue cells of sponge infested brown mussel were hypertrophy of the infested nucleus, loss of chromatin; proliferation and hypertrophy of rough and smooth endoplasmic reticulum; (Plate 4, Figure A) and formation of small vesicles in nucleoplasm of degenerating nuclei of cells in large numbers of cells (Plate 4, Figure C).

Plate 2 A-D: Electron micrographs of uninfected mantle tissue of Perna indica.
N: Nucleus x 20,000 B, EC: Endocytic Canals; M: Mitochondria (M) x 40,000 C;
ER: Endoplasmic reticulum (ER) x 40,000 D; ER: Endoplasmic reticulum (ER) x 50,000
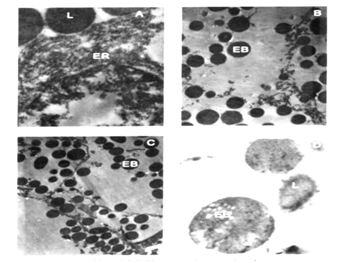
Plate 3 A-D: Electron micrographs of sponge infested mantle tissue of Perna indica.
A: Fragmented endoplasmic reticulum (ER), lysosomes (L) x 50,000
B: Cellular dystrophy showing the presence of only electron dense bodies (EB) x 40,000
C: Electron dense bodies migrating towards the peripheral region of cell (EB) x 40, 000
D: Magnified view electron dense bodies (EB) x 50, 000
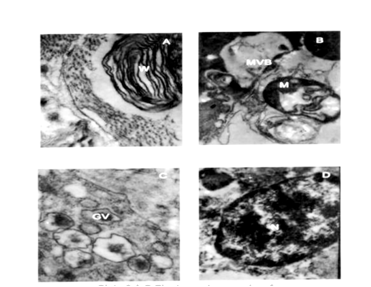
Plate 4 A-D: Electron micrographs of sponge infested mantle tissue.
A: Whorl formation (W) x 20,000.
B: Engulfing mitochondria (M), multivesicular body (MVB) x 40,000
C: Golgi vesicles (GV) x 40,000
D: Degenerating nucleus (N) x 25, 000
Studies on histology of sponge infested tissue revealed the infiltration of haemocytes into the mantle connective tissue lying adjacent to the epithelial cells underlying the region of calcareous material. The histopathological progression of haemic neoplasms in bay mussels showed cellular disorders in the connective tissue, mantle which are characterized by large, multiple and pleomorphic nuclei.5 The mantle areas, which normally secrete nacre, secrete periostracum under abnormal conditions like disease or shell damage. Under these circumstances the mantle shows remarkable changes like development of elongated cells, infiltration of haemocytes etc.4 Prokaryotic infestation in the sea scallop, Placopectenmagellanicus, resulted in grayish, flaccid adductor muscles with prominent my degeneration.3 It is possible that accumulation of intra cellular secretions is the result of increased cellular activity induced by stressors. No marked pathological change was observed in the mantle tissue of Chicoreusramosus infested by sporocysts of trematodes and nematodes.6 The cells of mantle epithelium showed structural changes and degeneration involving atrophy of cells. Bubel2 stated that periostracum repair in damaged cells is similar to that in cells infested by parasites. The ultra structure of the neopilinid mantle edge differs from that of other bivalves in several aspects. The periostracum groove is in fact situated between the inner surface of the median mantle fold and the weakly developed inner mantle fold, a condition being not found in any other molluscan group.9
Immediately after shell damage, the cells undergo increase in secretory activity. It in turn results in increase in the number of lysosomes and multivesicular bodies leading to the formation of an organized layer called proto-ostracum.2 In the present study also electron dense bodies were observed throughout the area examined for ultra structure changes. Ultra structure studies on the mantle tissue of Pinctada margaritifera revealed the presence of distinct epithelial areas; inner pallial zone made of columnar epithelium, middle fold made of cuboidal or ciliated cells, periostracal groove consisted of pseudo stratified epithelium and outer pallial zone of cuboidal epithelium.8 Electron microscopy has revealed the extent of damage caused due to the infestation of boring sponges in Perna indica. It resulted in a series of immunological changes; the prominent among them are increased cellular autophagy, haemocytosis, hypertrophy of nucleus and endoplasmic reticulum, vesicle formation in nucleolus and whorl formation of mitochondria. Even though sponge infestation seems to be superficial in mollusks severe infestation causes drain of energy which affects normal growth and other processes like pearl formation in the case of pearl oysters.
None.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.