eISSN: 2574-9838


Opinion Volume 8 Issue 3
Advanced Polymeric Nanostructured Materials Engineering, Graduate School of Engineering, Toyota Technological Institute, 2-12-1 Hisakata, Tempaku, Nagoya 468 8511, Japan
Correspondence: Masami Okamoto, Advanced Polymeric Nanostructured Materials Engineering, Graduate School of Engineering, Toyota Technological Institute, 2-12-1 Hisakata, Tempaku, Nagoya 468 8511, Japan, Tel +81 52 809 1861, Fax +81 52 809 1864
Received: November 22, 2023 | Published: December 8, 2023
Citation: Okamoto M. Viscoelasticity effect of the substrate to mimic microenvironment of breast cancer cells. Int Phys Med Rehab J. 2023;8(3):215-218. DOI: 10.15406/ipmrj.2023.08.00362
breast cancer, metastasis, epithelial-mesenchymal transition, cancer stem cells, viscoelastic properties, microenvironment
CSC, cancer stem cell; EMT, epithelial-mesenchymal transition; tan, damping coefficient; ECM, extracellular matrix; AC, acrylamide-based copolymer; MCF-7, breast adenocarcinoma cell line; G¢, storage modulus; TCPs, tissue culture plates; ALDH, aldehyde dehydrogenase; CTCs, circulating tumor cells
Metastasis is one of the biggest issues in cancer treatment today. Mammary epithelial cells are supported in optimal by interaction with a soft matrix (microenvironment) with an elastic modulus of approximately 800 Pa.1 On the other hand, after transformation, the breast tissue becomes progressively stiffer, and the tumor cells shrink significantly and become hypersensitive to the elasticity of the matrix. Additionally, and importantly, cancer cells invade blood vessels and enter the circulation during metastasis. The elastic modulus of fluids such as blood and mucus is approximately 50Pa, which is a very low stiffness. Therefore, it should be emphasized that the important association between cancer cell phenotype and changes in matrix stiffness is orders of magnitude smaller. This opinion focuses on the current understanding of cancer stem cells (CSCs) and epithelial-mesenchymal transition (EMT) in metastasis and identified the importance of research for engineered extracellular matrices with different viscoelastic properties to mimic the in vivo microenvironment.
Breast cancer has the most common incidence and mortality rate among malignant tumors in women in Japan.2 More than 90% of deaths from breast cancer are due to metastasis, primarily to the bones, lungs, liver, and lymph nodes. A complex process containing a series of discrete steps of metastasis is shown in Figure 1: (a) EMT, a period during which cancer cells lose cell-cell contacts and gain motility; (b) local tissue invasion promoted by extracellular matrix (ECM) degradation; (c) in blood vessels, where cancer cells penetrate the blood vessel wall and enter the circulation; (d) homing to bind platelets, during which cancer cells must survive in the circulation; (e) extravasation of cancer cells through blood vessel walls and out of the bloodstream of distant organs; (f) forms a metastatic niche at the metastatic site, creating an environment favorable for cancer cell proliferation.3
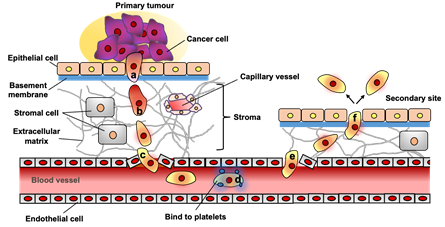
Figure 1 The several steps of metastasis. (a) epithelial-mesenchymal transition (EMT), during which cancer cells lose cell-to-cell contact and gain motility; (b) local tissue invasion, which is facilitated by the degradation of extracellular matrix (ECM); (c) intravasation, during which cancer cells penetrate the wall of a blood vessel and enter the circulation;(d) homing that bind to metastasis-supporting sites or to platelets, during which cancer cells must survive within the circulation; (e) extravasation, during which cancer cells pass through the vascular wall and exit the blood stream at distant organs; (f) metastatic niche formation at the metastatic site to create a milieu that is favorable for cancer cell growth.3
The average overall survival rate in 5-year of breast cancer is approximately 55%, due to poor radio- and chemotherapy-resistant treatment outcomes for metastatic disease.4 The poor prognosis of breast cancer treatment prompts consideration of effective cancer treatments. This means that breakthroughs in cancer treatment are essential.
Okamoto's research group has developed a hydrogel matrix of polymers (acrylamide-based copolymer having type I collagen: AC) with different viscoelasticity from 30 to 2x109 Pa as a microenvironment for cell culture substrates that influences cancer progression and metastatic potential. They investigated the viscoelasticity effect on the direct relationship between EMT-induced cell motility and mesenchymal properties in breast adenocarcinoma cell lines (MCF-7).5
The viscoelastic properties of substrates (polymer gels and tissue culture plates (TCPs)) are characterized by tan (and storage modulus (G¢). Viscoelastic materials generally stiff materials exhibit high stiffness and low loss, while soft materials have high damping and low stiffness. Compliant tissues such as the lungs exhibit low stiffness (~300 Pa). Bones and skeletal muscle exposed to high mechanical loads exhibit high stiffness by four orders of magnitude (104–106 Pa). In contrast, mucus and blood exhibit very low elastic moduli of 50 Pa.6,7
CSCs, a subpopulation of cancer cells, have been shown to exhibit stem cell characteristics that influence tumorigenesis8. These CSCs possess various cancer-promoting properties, including self-renewal differentiation, chemoresistance, and metastatic potential.8 CSCs have been shown to express various markers of CSC, such as CXCR4, CD44, and CD133 and, or to be enriched in cells with high aldehyde dehydrogenase (ALDH) activity. CD133 expression is inversely associated with poor 5-year overall survival rate and disease-free survival in cancer patients and is therefore a strong predictor of poor prognosis.9 The cell surface glycoprotein CD44, putative CSC marker which has been reported to be an adhesion molecule expressed on CSCs. Upregulation of CD44 enhances tumor growth and antiapoptotic effects.10
In comparison, the expression levels of each substrate tested in this study are plotted as a function of tand Figure 2.11 CD133+CD44– levels increase with increasing tand up to approximately 0.09 and a decreasing trend in cells cultured on AC-soft (G¢=32 Pa) substrate under both oxygen concentration conditions Figure 2a. CD133–CD44+ levels are driven by the damping feature under normoxic and hypoxic conditions. In normoxia, levels of CD133–CD44+ in cells cultured on AC soft substrate shows 250 times higher as compared to cells cultured on AC-s/o (G¢=2512 Pa), suggesting that a large amount of surface molecules of CD44 is produced in the soft gel matrix Figure 2b. These findings indicate that CD133–CD44+ expression in MCF-7 is uncoupled from CD133+CD44– expression under different oxygen concentration levels.
Regarding co-expression of CD133+CD44+, more stem-like characteristics seem to be affected by both CD133–CD44+ and CD133+CD44– combinations compared to other populations, i.e., further increase in the levels of cells incubated with AC-soft (tand=0.244, G¢=32 Pa) via the AC-mid (tand=0.193, G¢=174 Pa) substrate is seen Figure 2c.
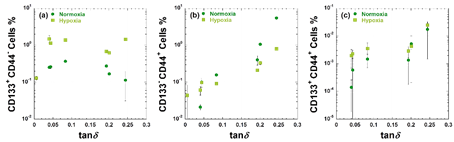
Figure 2 Relationships between CD133+CD44–(a), CD133–CD44+ (b), and CD133+CD44+ (c) expression and damping coefficient (tan) for MCF-7 at day 7 cultured on six different AC gel substrates and TCP-coat under both normoxic and hypoxic conditions.11
Until now the introduction of EMT is believed to facilitate CSC functionality. The CSC theory is widely accepted as a central principle explaining tumor aggressiveness, recurrence, chemoresistance, and even metastasis due to EMT phenomena.12,13 A hyaluronan receptor, CD44 is to promote cell adhesion and assembly of cell surface growth factors, particularly in maintaining cell-matrix interactions and maintaining the stem cell phenotype.14 CD44 has been identified as the main component. However, the mechanism as to how tand feature controls CD44 expression is unclear in terms of regulation of a wide variety of signaling pathways.
In clinical practice, studies have shown that circulating tumor cells (CTCs) in sufferers with metastatic breast cancer frequently express mesenchymal markers, whereas mesenchymal markers are only found in rare cells within the corresponding primary tumor. Especially, it has also been shown that the majority of CTCs found in blood samples of breast cancer patients also express the stem cell marker ALDH.15 For this reason, breast cancer cells, with or without EMT in the endovascular circulation, can induce CSC properties by an inducing microenvironment of quite low modulus (~50 Pa) see Figure 2b. This fact indicates that MCF-7 incubated on softer substrates can lead to the expression of CSC biomarkers indicative of higher CD44 expression.
Aiming the viscoelasticity of the microenvironment around cancer appears to represent a new direction for cancer therapy. Deep understanding not only the cancer cells themselves, but also the relationship between cancer cells and the viscoelastic microenvironment such as stroma and intravascular circulation are imperative. The role of viscoelastic properties in the microenvironment is only beginning to be explored.16–18 Most important is the realization that we may have the necessary tools (tand feature) to achieve eradicating and treatment with the ultimate goal of the treatment of cancer.
This work was supported by the KAKENHI of the Ministry of education, Sports, science and Technology, Japan.
Author declares that there are no conflicts of interests.

©2023 Okamoto. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.