International Journal of
eISSN: 2475-5559


Review Article Volume 1 Issue 3
1State Key Laboratory of Oil and Gas Geology and Exploitation, China
1State Key Laboratory of Oil and Gas Geology and Exploitation, China
2School of Geoscience and Technology, SWPU, China
2School of Geoscience and Technology, SWPU, China
Correspondence: Hong qi liu, State Key Laboratory of Oil and Gas Geology and Exploitation, China
Received: August 08, 2016 | Published: November 11, 2016
Citation: Liu HQ, Tian J, Youming D, et al. Study of the low-frequency dispersion of permittivity and resistivity in tight rocks. Int J Petrochem Sci Eng. 2016;1(3):55-61. DOI: 10.15406/ipcse.2016.01.00011
This study investigated the frequency dispersion of the resistivity and permittivity in tight rocks of the Daanzhai Group, Jurassic formation, Sichuan Basin. Tight limestone cores were used in the experiment, and the frequency varied from 0.1Hz to 1kHzunder laboratory conditions. The permittivity and resistivity decrease as the frequency increases, but the changing rates of these two parameters are different, as the permittivity exhibits a more obvious dispersion than the resistivity at both low and high frequency. Over the whole frequency range, the change rate of the resistivity is very small and can even be neglected. The change rate of the permittivity, however, is very significant, and its difference reached several orders of magnitude. Besides this phenomenon, the frequency dispersion degree increases as the water saturation and concentration increase. Therefore, we establish a group of equations to describe this relationship between the frequency dispersion and water saturation, and water concentration. Unlike Archie’s equation, which need multiple experimental parameters, the new formula can directly calculate the saturation from RP and CP or from DR and DC, only requiring two variables. Furthermore, frequency scanning test still implicates abundant information, which perhaps characterizes the pore structure, porosity, permeability and other properties of rocks or formations.
Keywords: Litho-electrical experiment; Resistivity; Permittivity; Frequency dispersion degree; Water saturation; Salinity; Tight rock
Resistivity and dielectric permittivity are important physical parameters that govern the propagation of electromagnetic fields (EMFs) in porous rocks. The resistivity of rocks has been researched for several centuries, and hundreds of thousands of studies have been published on them.1 First presented his litho-electric experiment results and provided an equation to relate the resistivity and saturation of rocks. Thereafter, one can not only qualitatively evaluate the fluids in rocks but also quantitatively calculate the amount of water and hydrocarbons. Generally, the resistivity and permittivity of the rock are functions of the chemical composition, density, porosity, water saturation, in homogeneity, polycrystallinity, lithology and component geometries, which in turn are related to the particle size distribution (or rock texture), rock bulk density, and shape of the rock particles.2-7 However, it is difficult to confirm the exact contribution of each physical and petro logical parameter to a sample’s measured dielectric constant. Of all of these aspects, the most dominant factor affecting the dielectric permittivity of rock is its water saturation, including both bound and free water.
The frequency dispersion of the complex dielectric constant provides useful information for geophysicists, petro physicists, and any person interested in rocks. Most published studies have focused on the properties of the dielectric constant at high frequency, usually in the range of kHzto MHz, or even GHz.8-14 However, the phenomenon of low-frequency dispersion (LFD), which is widely observed in many materials, was not been properly recognized until its discovery by the Chelsea Dielectrics Group15 and the investigation of its properties with increasing precision, as described in the book Dielectric Relaxation in Solids (DRS). Earlier studies proved that most rocks and minerals show dielectric dispersion in the low-frequency region from a few Hz to several MHz.16-18 At low frequencies, the dielectric dispersion of geological materials is believed to be caused by the polarization associated with charge buildup at the grain boundaries or grain imperfections. However, grain sizes and their distribution also control the frequency-dependent ε’ values of the geological samples.19,20 Many studies confirm that sandstones and other geological materials exhibit22 dielectric dispersion at low frequencies.16,20,21,23-27>
Although the Cole-Cole model is a relatively universal equation to depict the polarization and relaxation process for most media, there are still many mechanisms that are not fully understood. Summarized three types of polarization in rocks, namely, electronic, orientational and interfacial, and they noted that the predominance of these regimes depends on the frequency of the external electric field. Each type of polarization will vanish past a certain frequency, which is determined by the inertial moment of the particles in question, frictions and electrostatic forces. They expressed that the electronic polarization, corresponding to rock permittivity, occurs at a range of 1Hz to approximately 1×108Hz; molecular orientation, corresponding to the water molecular permittivity, occurs at range of 1Hz to approximately 1×1010Hz; and interfacial polarization, corresponding to pore geometry and ions, occurs at a range of 1Hz to approximately 1×1016Hz.
Obviously, in the low-frequency domain, the dielectric permittivity dispersion bears abundant information about the matrix, shale, fluid and pores, and even the geometry of the pores, their distribution, and the connectivity of the pore throat. For the case of tight reservoirs, a low frequency should be more useful to disclose the mysteries hidden in the rocks. This paper presented an experiment on tight limestone from Daanzhai Group, Jurassic formation, Sichuan Basin, and discussed the results in detail. In this paper, we mainly focused on the characteristics of the permittivity dispersion and its relationships with the saturation and concentration. In addition, we established a group of equations to describe the relationships of the frequency dispersion with the resistivity and permittivity. We defined two variables, DR andDC, to depict the decreasing rate of RP and CP. Based on these two variables, we established a formula to calculate the saturation of samples.28
General description of experiment
We selected tight lime stones out of 18 different lithological rocks, as shown in Figure 1. The specifications of it were listed in Table 1. To simulate the environment of the borehole, unlike usual cores, the samples were 15cm height, 10cm in diameter, and with a hole in middle of the cylinder, named the mid-hole, with a diameter of 1.5cm, through which the electrodes were placed while measuring. Artificial mud was also injected into this hole to assure that the electrical current can penetrate into the core.
No |
Height |
Diameter |
Mid-Hole Diameter |
Dried Weight |
Volume |
Porosity |
Permeability |
16 |
15.00 |
10.47 |
1.43 |
3048 |
1266.71 |
5.90 |
0.013 |
Table 1 Basic parameters of tight rock sample.
We used a LCR Analyzer to detect the resistivity and dielectric permittivity, and BH-II high pressure vacuum saturation device to saturate samples with different concentrations of NaCl solute.
We selected carbon fiber as electrodes, which show high electrical stability even in high-concentration salt solutions. First, the artificial mud was injected into the mid-hole, and then the electrodes were placed into the mid-hole. The mud can conduct the current into the core so that the electromagnetic field would be almost homogeneously distributed around the borehole at a relative shallow distance from the mid-hole. This configuration is very similar to real borehole environment of well logging operations.
Experiment processing
First, we prepared three different concentrations of NaCl solution (CW): 0.125mol/L, 0.25mol/L, and 0.5mol/L. Secondly, the samples were dried in microwave oven; and then they were put into a container of BH-II, and saturated with NaCl solution of different CW. Subsequently, the electrode was placed into the mid-hole of the cores, and the last step was to switch on LCR Analyzer, the resistivity and permittivity could be shown on the screen together. The LCR can provide these two parameters Rs, C sin serial and RP, CP, in parallel modes, respectively, where the subscript “s” means series mode, and “p” indicates a parallel mode. For actual situation, generally, borehole is divided into flushed zone, transition zone and uncontaminated zone. The current conducts through these three zones in parallel, so during the experiment, we measured RP and CP in different saturation and NaCl concentration, and the frequency ranged from 0.1Hz to 1kHz. Table 2 listed the dry weight, saturation and concentration of NaCl solution.
0.125 |
Dry weight/g |
3172.0 |
3128.0 |
3108.0 |
3094.0 |
3050.0 |
Saturation/% |
88.36 |
56.49 |
42.01 |
31.87 |
0.00 |
|
0.25 |
Dry weight/g |
3178.0 |
3138.0 |
3116.0 |
3070.0 |
3050.0 |
Saturation/% |
92.71 |
63.74 |
47.80 |
14.49 |
0.00 |
|
0.5 |
Dry weight/g |
3182.0 |
3148.0 |
3116.0 |
3084.0 |
3050.0 |
Saturation/% |
95.60 |
70.98 |
47.80 |
24.62 |
0.0 |
Table 2 Water content, saturation and dry weight of Sample.
Measurement of resistivity and permittivity
During this experiment, we tested the sample with five different saturation, three different concentration, and frequency ranged from 0.1Hz to 1 kHz (the increment is on a logarithm scale), so totally 15 group, each 105 data points of resistivity and permittivity were recorded. Part of the data were presented in Table 3, in which RP1,RP2,…,RP5, correspond to resistivity in parallel in five different saturation, and CP1,CP2,…,CP5 correspond permittivity in parallel. Data in concentration of 0.25mol/L and 0.5mol/L were not listed out take account of the length of the paper Table 3.
F/Hz |
RP1 |
RP2 |
RP3 |
RP4 |
RP5 |
|---|---|---|---|---|---|
Sw1=88.36% |
SW2=56.49% |
SW3=42.01% |
SW4=31.87% |
SW5=0% |
|
0.1 |
1.18E+03 |
1.27E+03 |
2.01E+03 |
2.62E+03 |
8.14E+03 |
24 |
8.38E+02 |
1.04E+03 |
1.61E+03 |
2.15E+03 |
6.88E+03 |
35 |
8.32E+02 |
1.03E+03 |
1.60E+03 |
2.14E+03 |
6.85E+03 |
155.6 |
8.10E+02 |
1.01E+03 |
1.57E+03 |
2.11E+03 |
6.77E+03 |
192 |
8.07E+02 |
1.00E+03 |
1.57E+03 |
2.11E+03 |
6.76E+03 |
225.7 |
8.05E+02 |
1.00E+03 |
1.57E+03 |
2.10E+03 |
6.75E+03 |
280 |
8.02E+02 |
9.99E+02 |
1.56E+03 |
2.10E+03 |
6.73E+03 |
628 |
7.93E+02 |
9.88E+02 |
1.55E+03 |
2.08E+03 |
6.69E+03 |
1000 |
7.88E+02 |
9.82E+02 |
1.54E+03 |
2.07E+03 |
6.66E+03 |
F/Hz |
CP1 |
CP2 |
CP3 |
CP4 |
CP5 |
SW1=88.36% |
SW2=59.49% |
SW3=42.01% |
SW4=31.87% |
SW5=0% |
|
0.1 |
5.10E-04 |
3.14E-04 |
2.05E-04 |
1.26E-04 |
2.58E-05 |
24 |
3.82E-07 |
2.46E-07 |
1.33E-07 |
8.12E-08 |
2.13E-08 |
35 |
2.43E-07 |
1.59E-07 |
8.50E-08 |
5.20E-08 |
1.38E-08 |
155.6 |
4.36E-08 |
3.02E-08 |
1.65E-08 |
1.05E-08 |
2.83E-09 |
192 |
3.45E-08 |
2.42E-08 |
1.31E-08 |
8.56E-09 |
2.30E-09 |
225.7 |
2.89E-08 |
2.05E-08 |
1.13E-08 |
7.24E-09 |
2.02E-09 |
280 |
2.29E-08 |
1.63E-08 |
9.06E-09 |
5.93E-09 |
1.65E-09 |
628 |
9.68E-09 |
7.14E-09 |
4.14E-09 |
2.79E-09 |
8.44E-10 |
1000 |
5.97E-09 |
4.49E-09 |
2.66E-09 |
1.84E-09 |
5.99E-10 |
Table 3 Part of resistivity data with concentration of 0.125mol/L (Ω•m).
Data analysis
We wanted to resolved four questions based on the above experimental results:
Characteristics of frequency dispersion: Based on the results of experiment, Figures 2-4 show the relationships of resistivity and permittivity’s.Frequency. Figure 2 exhibits five curves, and each one corresponded to different saturation that listed in Table 2. All of these 5 curves were with concentration of 0.125mol/L. Figure 3 shows similar curves corresponding concentration of 0.25mol/L, and Figure 4 shows curves with concentration of 0.5mol/L. From these figures, we can summarize the following laws:
a. Frequency dispersion of resistivity b. Frequency dispersion of permittivity
a. Frequency dispersion of RP b. Frequency dispersion of CP
a. Frequency dispersion of RP b. frequency dispersion of CP
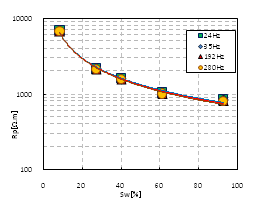
Based on these experimental data, we have established a set of new formulas between RP and CP and frequency, as follows.
CW= 0.125mol/L
SW1=88.36%:RP1=958.57×F-0.035(R2=0.8823), CP1=2×10-5×F-1.239(R2=0.9983);
SW2=59.49%:RP2=1134.7×F-0.025 (R2=0.9335), CP2=1×10-5×F-1.237(R2=0.9976);
SW3=42.01%:RP3=1758.1×F-0.024 (R2=0.8875), CP3=8×10-6×F-1.233(R2=0.9965);
SW4=31.87%:RP4=2319.3×F-0.020 (R2=0.8758), CP4=5×10-6×F-1.224(R2=0.9955);
SW5=0%:RP5=7310.2×F-0.017 (R2=0.8710), CP5=1×10-6×F-1.192(R2=0.9952);
Formulas for concentrations of 0.25mol/L and 0.5mol/L had similar exponent form, and were omitted for sake of concise paper. To describe the mathematical relation, we defined two variables.
A general equation for RP and CP, based on the above formulas, can be rewritten as follows:
(1)
(2)
Where A and B are constant, which can be got through least-square method.
It is well known that Debye presented a dielectric model in 1912, namely Debye Model, to clarify the relation of dielectric and frequency,22 has more extensive adaptability. Comparing to these two classical models, equation (2) is much easier and succinct.
Relationship between saturation and RP, CP: From the above figures and discussion, we can conclude that although the measurement frequency is different, RP and CP illustrate almost the same trend of variation as functions of the saturation and concentration. We therefore established certain equations to directly calculate the saturation using RP or CP, as shown in Figure 5 & 6. Comparing these two figures, we find that at the same saturation, resistivity has nearly the same value at different measurement frequencies; as for CP, however, has different values at different frequencies.
F=24Hz, SW = 44257RP-0.896 R² = 0.9931, SW = 2E-09CP1.2144 R² = 0.9978
F=35Hz, SW = 44285RP-0.897 R² = 0.9933, SW = 1E-09CP1.2108 R² = 0.9974
F=192Hz,SW = 44340RP-0.904 R² = 0.9937, SW = 2E-10CP1.1455 R² = 0.9974
F=280Hz,SW = 44303RP-0.905 R² = 0.9937, SW = 2E-10CP1.1114 R² = 0.9973
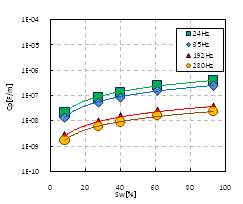
In addition to the RP and CP, we should also note that DR and DC are different for different saturations, and the values of these two parameters at different saturations and concentrations are listed in Table 4. The general rule is that the values are proportional to the saturation. The difference between DR and DC is that the latter is much larger than the former at the same saturation by approximately two orders of magnitude.
Para. |
SW1 |
SW2 |
SW3 |
SW4 |
SW5 |
DR |
0.035 |
0.025 |
0.024 |
0.02 |
0.017 |
DC |
1.239 |
1.237 |
1.233 |
1.224 |
1.192 |
Table 4 DR and DC with different saturations at 0.125mol/L.
This means that there is some implicit relationship between DR, DCand the saturation. Figure 7 shows the relationship of SW vs. DRat concentrations of 0.125mol/L, 0.25mol/L, and 0.5mol/L, and Figure 8 shows the relationship of SW vs. DCat concentrations of 0.125mol/L, 0.25mol/L, and 0.5mol/L.
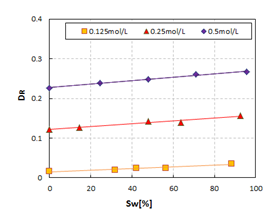
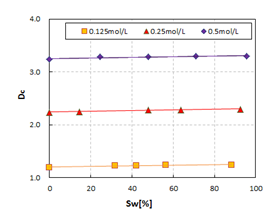
Based on these two figures, we can see that the saturation can be calculated from DRand DC, in accordance with the below equations. The mechanism of this phenomenon will be discussed in another paper.
CW=0.125mol/L: SW=4589.0DR-67.308, R2=0.9343, SW=1485.4DC-1775.8, R2=0.7828;
CW=0.250mol/L: SW=2652.2DR-321.20, R2=0.9540, SW=1444.0DC-3233.9, R2=0.9123;
CW=0.500mol/L: SW=2255.9DR-512.11, R2=0.9871, SW=1460.5DC-4744.0, R2=0.7826.
These equations proofed that frequency dispersion can be used to calculate saturation.
Through the measurement of RP and CP of tight rock sample under different saturation and concentration in the frequency range from 0.1Hz to 1kHz, the properties of RP and CP were investigated in detail. From the results of this experiment, we have discovered some characteristics of the frequency dispersion of resistivity and permittivity. Furthermore, a set of equations and variables to depict the dispersion laws have been constructed in this paper.
We summarize the findings as follows:
The authors are grateful for the financial support from the Basic Science Program of Advanced Well Logging Technology of CNPC (2014A-2319) and the Project of Science and Technology Program (G12-3) of the State Key Lab of Oil and Gas Reservoir Geology and Exploitation of SWPU. We also thank the graduate students Zhou Guangzhao, Xiang Tai, and Feng Hui for their cooperation in this research; they plotted many of the figures and helped edit this paper. The authors are extremely grateful to Professor Zhao Liangxiao, Southwest Oil and Gas Company, for his constructive advice.
F : Frequency, Hz.
CW: Concentration of NaCl solution, mol/L;
CP: Permittivity, F/m;
RP: Resistivity, Ω·m;
K: Electrode coefficient, cm;
SW: Saturation of water;
DR: Dispersion exponent of resistivity, dimensionless;
DC: Dispersion exponent of permittivity, dimensionless;
A: Coefficient of resistivity, Ω·m;
B: Coefficient of permittivity, F/m;
The author declares no conflict of interest.

©2016 Liu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.