International Journal of
eISSN: 2475-5559


Research Article Volume 1 Issue 1
1SINOPEC Research Institute of Petroleum Engineer, China
1SINOPEC Research Institute of Petroleum Engineer, China
Correspondence: Zeng Yijin, SINOPEC Research Institute of Petroleum Engineer, China
Received: August 02, 2016 | Published: August 19, 2016
Citation: Yijin Z, Shiming Z. Study of corrosion mechanism of sour gas to cement stone in PUGUANG gas field. Int J Petrochem Sci Eng. 2016;1(1):4-8. DOI: 10.15406/ipcse.2016.01.00002
The gas reservoir of PUGUANG contains high levels of H2S and CO2 with 15% and 8% by volume respectively. As to the corrosion of CO2/H2S mixture to cement, people seldom involve in research. Cement stone samples corroded by H2S/CO2 mixture under different temperature and pressure are tested to probe the change of compressive strength and permeability. Microstructure and corroded products of corroded samples were observed by SEM and XRD. The result shows the corroded products of CO2/H2S mixture to cement are similar to those by single-component H2S or CO2 gas, except that the amount of expansive crystal produced by H2S is reduced. Combination of H2S and CO2 accelerates the corrosion progress, the recession of strengthen and permeability is more serious than that of single action by H2S or CO2 simultaneously, but CO2 dominates the whole corrosion process after the long duration. Fly ash and Clay have benefits to resist corrosion of combination of H2S and CO2.
sour gas, cement stone, corrosion, H2S, CO2, puguang
The PUGUANG gas field is the biggest sour gas field in China which contains 15% and 8% by volume of H2S and CO2 respectively.1Acidic gas and alkaline cement ring will react in wet condition to cause compressive strengthen reduce and damage seal function of cement ring.2 ̶ 3 Previous studies focused on the single corrosion mechanism of CO2 or H2S to cement stone, as to the corrosion of CO2/H2S mixture to cement, people seldom involve in research. Such as Yaoxiao4 & Zhou Shiming5 has studied the change of compressive strengthen of cement by CO2, Ma kaiHua6 has made systematic studies on the H2S corrosion to cement. What will happen on the cement after the combinatorial action by H2S and CO2 mixture? In this paper test methods are established under well whole condition of PUGUANG gas field,7 the compressive strength and permeability of cement stone are measured before and after corrosion according to API Spec10B. SEM and XRD are used to study the change of micro structure and reaction product before and after corrosion.
Experimental process
Experimental parameter: Test parameters are determined according to well condition of PUGUANG gas field as follows:
Experimental process
Slurry is prepared according to API Spec10B and poured into test mold and cured in HTHP cell for 24hours. The half of set samples are removed from molds and numbered and putted into the corroding chamber and cured for 21days, then seal the chamber and inject H2S & CO2 mixture and apply heat and pressure according to required temperature and CO2 & H2S partial pressure. The rest of samples are cured in HTHP cell as comparative analysis samples. During the test the temperature and pressure in the chamber are hold constant at all time by refilling mixture gas and auto temperature controlling.8
The compositions of slurry
Two groups of slurry composition are prepared for test listed in Table 1 & 2. The ingredients of group 2 contain 35% silica by weight of cement when the temperature is more than 110˚C. The number of composition matches the number of sample in the following text.
Composition No. |
1 |
2 |
3 |
4 |
5 |
6 |
API G class cement, g |
500 |
500 |
500 |
500 |
600 |
500 |
Silica flour, g |
- |
- |
- |
- |
- |
175 |
Dispersant, g |
- |
7.5 |
17.5 |
15 |
17.5 |
- |
Filtration controller, g |
- |
30 |
30 |
30 |
30 |
- |
Latex, g |
- |
60 |
60 |
- |
60 |
- |
Al2O3, g |
- |
- |
- |
50 |
- |
- |
Clay, g |
- |
- |
50 |
- |
50 |
- |
Fly ash, g |
- |
- |
90 |
150 |
75 |
- |
Table 1 Slurry compositions of group 1 at 95˚C.
Composition No. |
1 |
2 |
3 |
4 |
5 |
6 |
APIG class cement g |
500 |
500 |
500 |
500 |
600 |
500 |
Silica flour g |
175 |
175 |
175 |
150 |
175 |
175 |
Dispersant, g |
- |
7.5 |
17.5 |
15 |
17.5 |
17.5 |
Filtration controller, g |
- |
30 |
30 |
30 |
30 |
30 |
Latex, g |
- |
60 |
60 |
- |
60 |
60 |
Al2O3,g |
- |
- |
25 |
50 |
25 |
25 |
Clay, g |
25 |
25 |
- |
25 |
- |
|
Fly ash, g |
75 |
75 |
150 |
75 |
75 |
|
Table 2 Slurry compositions of group 2 at 130˚C and 150˚C.
Change of compressive strengthen and permeability after corrosion
Table 3 shows the change of compressive strengthen and permeability of group 1 cement stone before and after corrosion at 95˚C, and Table 4 & 5 show those changes of group 2 at 130˚C and 150˚C. Table 3 ̶ 5 shows most of samples present compressive strengthen reduction and permeability increase after corrosion. Sample 3 and 4 show comparatively good corrosion resistance with other samples.
Information in tables also shows that as the temperature rises, the recession of strengthen and permeability is more serious, which presents a complete opposite rule with that of single corrosion by H2S or CO2.
Number of Sample |
Compressive Strength (Mpa) |
Permeability K/(10-3um2) |
||||
|---|---|---|---|---|---|---|
Before Corrosion |
After Corrosion |
Change |
Before Corrosion |
After Corrosion |
Change |
|
% |
% |
|||||
1 |
17.01 |
18.82 |
10.64 |
0.2006 |
0.3317 |
65.35 |
2 |
13.83 |
12.13 |
-12.29 |
0.2978 |
0.3351 |
12.53 |
3 |
13.15 |
11.79 |
-10.34 |
0.1903 |
0.3134 |
64.69 |
4 |
14.23 |
14.29 |
0.42 |
0.3 |
0.2743 |
-8.57 |
5 |
18.71 |
14.52 |
-22.39 |
0.1677 |
0.2714 |
61.84 |
6 |
20.47 |
16.33 |
-20.22 |
0.2 |
0.279 |
39.5 |
Table 3 Change of compressive strengthen and permeability after corrosion at 95℃.
Number of Sample |
Changes in Strength (Mpa) |
Permeability K / K/(10-3um2) |
||||
|---|---|---|---|---|---|---|
Before Corrosion |
After Corrosion |
Change |
Before Corrosion |
After Corrosion |
Change |
|
% |
% |
|||||
1 |
25.6 |
15.2 |
-40.63 |
0.623 |
2.731 |
338.36 |
2 |
18.48 |
13.38 |
-27.6 |
0.621 |
2.745 |
342.03 |
3 |
14.86 |
16.22 |
9.15 |
0.439 |
0.362 |
-17.54 |
4 |
18.37 |
17.69 |
-3.7 |
0.417 |
0.522 |
25.18 |
5 |
13.5 |
15.4 |
14.07 |
0.384 |
3.337 |
769.01 |
6 |
14.29 |
14.29 |
0 |
0.556 |
2.923 |
425.72 |
Table 4 Change of compressive strengthen and permeability after corrosion at 130℃.
Number of sample |
Change in strength (Mpa) |
Permeability K / K/(10-3um2) |
||||
|---|---|---|---|---|---|---|
before corrosion |
after corrosion |
Change |
before corrosion |
After corrosion |
Change |
|
% |
% |
|||||
1 |
38.73 |
21.05 |
-45.65 |
0.3255 |
0.523 |
60.68 |
2 |
32.34 |
13.56 |
-58.07 |
0.2969 |
2.755 |
827.92 |
3 |
17.18 |
17.46 |
1.63 |
0.2988 |
0.672 |
124.9 |
4 |
31.58 |
25.86 |
-18.11 |
0.312 |
1.828 |
485.9 |
5 |
15.93 |
14.23 |
-10.67 |
0.3669 |
0.4561 |
24.31 |
6 |
20.58 |
15.52 |
-24.59 |
- |
- |
- |
Table 5 change of compressive strengthen and permeability after corrosion at 150℃.
Analysis on reaction products of corroded cement samples
Corroded products analysis at 95˚C: Figure 1 is XRD result of sample 1 and Figure 2 is SEM picture of sample 1and 6 of group1. Figure 1 shows that there is lots of CaCO3 crystal in outer layers of sample 1, which are the products of CO2 reaction. Little CaSO4 Crystal is founded in the inside of the sample which is the products of H2S A large amount of Ca (OH) 2 is founded in the inner of sample 1. Figure 2 shows that there are lots of cracks and pores in both samples of 1 and 6 which verifies the recession of strengthen and permeability. The picture also indicates there is almost no hydrated calcium silicate (CSH) in the cement stone.
Corroded products analysis at 130℃: Figure 3 & 4 are of XRD results of the corroded samples from No.1to NO.6 of group 2 at 130˚C. Figure 3 analyzes products in the outer layer of samples and Figure 4 analyzes products of in the core of samples. Figure 3 shows there are large amount of mini-crystal calcium carbonate (CaCO3 (I)) and calcite (CaCO3 (II)) and a little gypsum in all the samples. Figure 4 shows almost no Ca(OH)2 in the core of samples. For lack Ca(OH)2, CSH lost stability by transforming to C2SH, which can explain why the recession of strengthen and permeability becoming more serious as the temperature rising. Figure 5 is the SEM pictures of sample 3 and 4. The picture indicates there are lots of cracks and pores in both samples. The picture also indicates There are almost no hydrated calcium silicate (CSH) and Ca(OH)2 in the cement stone
Corroded products analysis at 150˚C: Figure 6 & 7 are of XRD results of the corroded samples from No.1to NO.6 of group 2 at 150˚C. Figure 6 analyzes products in the outer layer of samples and Figure 7 analyzes products of in the core of samples. Figure 6 shows there are large amount of mini-crystal calcium carbonate (CaCO3 (I)) and calcite (CaCO3 (II)) and a little gypsum in all the samples. Figure 7 shows there is CaCO3 in the core of cement stone, which indicates the corroding reaction makes deeper. Figure 8 is the SEM pictures of sample 3 and 5. The picture indicates there are lots of cracks and pores in both samples, and almost no hydrated calcium silicate (CSH and Ca(OH)2 in the cement stone. The crystal of CaCO3 and CaSO4 is founded in the core of cement which verifies the further corroding reaction with temperature rising.
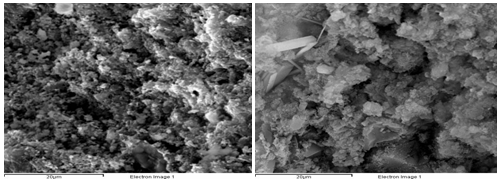
Outer layer SEM photos of No.3 and No.5 corroded sample (the left is No.3).
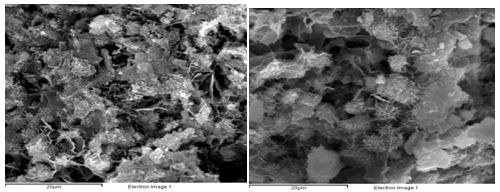
Inner layer SEM photos of No.3 and No.5 corroded sample (the left is No.3)
Figure 2 Core and outer layer SEM photos of No.1 and No.6 sample.
Corrosion mechanism of CO2 to cement complies with reaction formula (1) and (2).7,8
CO2 + H2O → H2CO3 → H+ + HCO3- (1)
Ca(OH)2 + H+ + H2CO3- → CaCO3 + 2H2O (2)
There is almost no CSH gel in the corroded cement stone sample, but there is large volume of C2SH, which shows that CSH gel begins to react with CO2 and produce CaCO3 and C2SH, its reaction complies with the reaction formula (3). 5,9 ̶11
CSH + H+ + HCO3- → C2SH + CaCO3 (3)
Firstly H2S reacts with Ca(OH)2 to produce CaSO4.2H2O (gypsum) and the volume of solid substance expands, producing fractures in cement stone, then it makes corrosion expanding into the cement until all cement gelatin is corroded and collapsed.
The reaction of H2S with cement stone is as following.12
Ca(OH)2(S) + H2S(g) + H2O(1) → CaSO4 + 2H2O (S) (4)
The density of Ca(OH)2 is 2.24 g/cm3, while the density of CaSO4·2H2O is 2.30 g/cm3. Therefore, when corroded by H2S, the volume cement stone will expand, and producing fractures in it. CSH gel of cement stone also reacts with H2S solution to produce CaSO4·2H2O (gypsum).
The reaction formula is as following.6
CSH + H2S + H2O → CaSO4·2H2O + C(m)S(n)H(x) (5)
CaSO4·2H2O will continues to react with C3A to produce Ettringite (AFT) catalyzed by Ca(OH)2,
The reaction formula is as following.12
C3A+3(CaSO4·2H2O) +2Ca(OH)2+24H2O-3CaO·Al2O3·3CaSO4·32H2O (6)
The density of Ettringite is 1.73g/cm3, too much ettringite generate will cause cement stone expanding split.
Corrosion mechanism of CO2 and H2S mixture to cement
The products corroded by H2S and CO2 mixture to Cement stone are similar to those by single-component gas.13 CO2 dominates the whole corrosion process in the long duration, because its’ corroding products are more than the products by H2S. For the small quantity of products by H2S, expanding split cannot be founded in whole process by combination of H2S and CO2.
Combination of H2S and CO2 accelerates the corrosion progress, the recession of strengthen and permeability is more serious than that of single action by H2S or CO2 simultaneously. As temperature rises, the recession of strengthen and permeability is more serious, which presents a complete opposite rule with that of single corrosion by H2S or CO2.
The temperature of bottom well bore of PUGUANG gas field is 150˚C,and the partial pressures of H2S and CO2 are 10MPa and 5MPa respectively, which will bring serious corrosion on cement ring, and damage its’ seal ability. The higher of the temperature rises, the more severe of the recession of strengthen and permeability, which presents a complete opposite rule with that of single corrosion by H2S or CO2. The composition of cement slurry is the predominant factor affecting cement corrosion resistance. The introduction of Latex and Fly ash and clay into system will reduce the alkalinity in the cement slurry system and improves the corrosion resistance of set cement. The hydration products are crystallized after corrosion under combination of H2S and CO2, and loose arrangement of that crystal is the reason of strength decline and permeability rise. Measures against composite corrosion of H2S and CO2 mixture: decline the alkalinity and reduce the porosity of cement slurry.
None.
The authors declare no conflict of interest.

©2016 Yijin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.